Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Clinical Samples Equipment Supplied In Europe
20 equipment items found
Manufactured by:BIORON Diagnostics GmbH based inRömerberg, GERMANY
RealLine Chlamydia trachomatis Kits detect Chlamydia trachomatis plasmid and genomic DNA in clinical samples by Real-Time PCR. The kits are designed for in vitro diagnostics and provide qualitative ...
Manufactured by:ECO MEDICS AG based inDuernten, SWITZERLAND
This kit offers a versatile alternative to onsite FeNO analyzers like the ANALYZER CLD 88 sp, allowing for efficient collection of exhaled breath samples in varied environments such as workplaces, schools, and clinics. Samples are gathered in a Mylar bag under specified conditions, enabling subsequent analysis with any compatible instrument. This ...
Manufactured by:Trina Bioreactives Ag based inNaenikon, SWITZERLAND
Any anticoagulant can be paired and matched ...
Manufactured by:OPTIMA packaging group GmbH based inSchwaebisch Hall, GERMANY
Transdermal patches and Oral film ...
Manufactured by:Coris BioConcept based inGembloux, BELGIUM
In vitro rapid diagnostic test for the detection of SARS-CoV-2 antigen in nasopharyngeal secretions. Since many months, several variants did pop-up from countries where the pandemic was particularly active (i.e. UK, Brazil, India and South Africa) and our test has been proven to detect all of ...
Manufactured by:bioMérieux S.A. based inMarcy l`Etoile, FRANCE
The NephroCheck Test Kit is a single-use cartridge designed to detect biomarkers of acute kidney injury, TIMP-2 and IGFBP-7. The test provides results in 20 ...
Manufactured by:Trina Bioreactives Ag based inNaenikon, SWITZERLAND
Over 50’000 donors for biospecimen from globally different diagnostic and demographic individuals. (Europe, Asia, Americas, Africa) . Different Matrixes (Serum, EDTA, LiHep, etc.) & Multibleeds 1 - 800 ml per Donor. Clinical Data: Patient Data (Age / Gender / GT) Detailed Anamnesis ...
Manufactured by:Genomtec SA based inWroclaw, POLAND
Saliva Sample Collector Device can be used for the collection, storage and transportation of native saliva sample for clinical diagnostic purposes and does not contain any nucleic acid stabilizing solution. It is DNaze / RNaze and pyrogen free and sterilized by radiation. ...
Manufactured by:A. Berents GmbH & Co. KG based inStuhr, GERMANY
The Laboratory Homogenising Mixer from BECOMIX is meticulously engineered to simulate large-scale production processes at a reduced scale, supporting various pharmaceutical and cosmetic applications. It offers shear variability, diagonal mixing, and hot/cold production capabilities, essential for creating high-quality emulsions and dispersions. The mixer is ideal for producing ointments, ...
Manufactured by:Yourgene Health based inManchester, UNITED KINGDOM
A CE-Marked RT-qPCR assay which detects the presence of SARS-CoV-2 viral ...
by:CHROMagar based inParis, FRANCE
CHROMagar™ Acinetobacter is a selective and differential chromogenic culture medium, intended for use in the qualitative direct detection of colonization with Acinetobacter to aid in the prevention and control of Acinetobacter, drug-susceptible or multi-drug resistant (MDR), in healthcare settings. The test is performed with rectal swabs, nare swabs, wound swabs, stools and urine ...
by:Diasorin based inSALUGGIA, ITALY
Sample-to-answer, real-time PCR systems designed for improved laboratory efficiency and workflow. ARIES and ARIES M1 Systems are Luminex’s sample-to-answer, real-time PCR systems that are crafted to increase laboratory efficiency, ensure result accuracy, and fit seamlessly into today’s lean ...
Manufactured by:Richen Europe S.r.l. based inTrezzano Sul Naviglio (MI), ITALY
The rapid antigen test has become the most used tool – both publicly and privately – to quickly find out if you have come into contact with the Sars-CoV-2 virus, thus allowing better tracking of infections. ...
Manufactured by:Genedrive Plc based inManchester, UNITED KINGDOM
7.5 minute1 point of care molecular testing now possible with the NEW CE marked Genedrive® COV19-ID ...
Manufactured by:Sysmex Partec GmbH based inGoerlitz, GERMANY
The new UC-3500 is a fully automated urine chemistry analyser that offers particularly high clinical value. Urine sample material is dropped onto each pad of a dedicated test strip within the analyser. The entire sequence, starting from sample aspiration, to colour comparison and final output of the results is fully automatic. In addition to the ...
Manufactured by:Roche Diagnostics International AG based inRotkreuz, SWITZERLAND
GenMark’s ePlex® Blood-Culture Identification (BCID) Panels provide broad coverage of organisms that can lead to sepsis along with their resistance genes. This broad coverage means that about 95% of currently identified bloodstream infections can be detected early with the ePlex BCID Panels, compared to other panels that detect significantly fewer sepsis-causing bacteria and fungi ...
Manufactured by:NZYTech - Genes & Enzymes based inLisboa, PORTUGAL
NZYTech´s COVID-19 & Flu A/B Multiplex One-Step RT-qPCR Kit (IVD) provides the complete set of reagents and probes to qualitatively detect the SARS-CoV-2 and/or Influenza genomes, through common real-time PCR platforms. SARS-CoV-2 is identified by detecting RT-qPCR targets located in RdRp and N genes. In contrast, Influenza A and Influenza B viruses are detected through the ...
Manufactured by:Genomtec SA based inWroclaw, POLAND
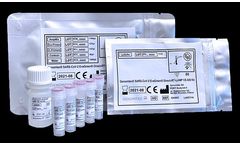
Genomtec® SARS-CoV-2 EvaGreen® Direct-RT-LAMP CE-IVD Kit is Real-Time Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) test for qualitative detection of nucleic acid from two genes of SARS-CoV-2 virus with a streamlined biological sample processing that is CE-IVD labelled for diagnostic use in the EU.* It features rapid RNA isolates preparation in conjugation with ...
Manufactured by:Destiny Pharma plc based inBrighton, UNITED KINGDOM
The XF drug platform has a novel, ultra-rapid mechanism that reduces the chance of bacteria becoming resistant to its action. Destiny Pharma’s XF platform has advantages over traditional antibiotics. ...
Manufactured by:Owlstone Medical Ltd. based inCambridge, UNITED KINGDOM
Owlstone Medical’s patented FAIMS technology can be applied to rapidly monitor a broad range of volatile compounds from breath, urine and other bodily fluids with high sensitivity and ...