Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Coronary Arterial Equipment Supplied In Europe
42 equipment items found
by:XELTIS BV based inEindhoven, NETHERLANDS
The XABG device is a restorative, synthetic, electrospun blood vessels for CABG surgery, overtime turning into a living vessel made of patient’s own ...
Manufactured by:Circle Cardiovascular Imaging Inc. based inCalgary, ALBERTA (CANADA)
Specialized tools for the assessment of coronary artery disease using cardiac CT. Read and report Cardiac CT with zero-click coronary artery segmentation and quantifying calcium scoring powered by ...
Manufactured by:Wellinq based inLeek, NETHERLANDS
High quality Angiographic catheter that provides reliable kink-free passage of tortuous anatomy and calcified lesions while keeping optimal torque control in the coronary ...
Manufactured by:Bioprojet based inParis, FRANCE
Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease having undergone a percutaneous coronary intervention (PCI), who have not received an oral P2Y12 receptor inhibitor prior to the intervention and in whom the oral ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
Novel, First of its kind Sirolimus drug coated balloon catheter for the treatment coronary artery disease. MagicTouch is intended for in-stent restenosis, small vessels, bifurcation lesions and de-novo lesions ...
Manufactured by:Celixir plc based inStratford-upon-Avon, UNITED KINGDOM
PHASE 2 : Our first investigational cardiac regenerative medicine consists of iMP cells. iMP cells are designed to treat patients with ischaemic heart disease – ischaemic cardiomyopathy – undergoing coronary artery bypass graft (CABG). They are injected around cardiac scar tissue via a single application during bypass ...
Manufactured by:CARDIONOVUM GmbH based inBonn, GERMANY
Unmatched XLIMUS clinical performance. The ultimate DES for complex artery lesions. XLIMUS is considered as the ultimate coronary DES stent system to treat complex coronary artery disease by reaching and crossing the most challenging ...
Manufactured by:KLS Martin Group based inTuttlingen, GERMANY
The new marTract retractor system for minimally invasive coronary artery bypass (MIDCAB) includes two individually adjustable traction devices. For sufficient stability, a large amount of force is no longer required when pulling the rib arches and the system is easily ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
ABLUMINUS DES+ designed by propriety ENVISOLUTION technology in a way that address the unmet clinical needs specially in Diabetes Mellitus and Acute Myocardial infarction patients with Coronary Artery Disease. ABLUMINUS DES+ Receives on-label indication for Diabetes Mellitus and Acute Myocardial infarction. ...
Manufactured by:amg International GmbH based inWinsen, GERMANY
The ARTHOSPico is a multicellular Cobalt-Chromium stent that offers strength, stability, and flexibility for treating the most difficult lesions. The Co-Cr Stent is indicated for patients with coronary heart disease. It is inserted in coronary vessels with a diameter of 2.0 to 4.0 mm. It is used for the treatment of de-novo and restenotic lesions in ...
Manufactured by:Swann-Morton Limited based inSheffield, UNITED KINGDOM
The No.10 blade with its curved cutting edge is one of the more traditional blade shapes and is used generally for making varying sizes of incision in skin and muscle. The No.10 is often utilised in more specialised surgeries such as for harvesting the radial artery during a coronary artery bypass operation, opening the bronchus during thoracic ...
Manufactured by:Carl Zeiss Meditec AG based inJena, GERMANY
INFRARED 800 from ZEISS enables intraoperative visual assessment of blood flow and vessel patency during arteriovenous malformation (AVM), bypass, aneurysm and anastomosis surgery. ZEISS INFRARED 800 is indicated for use in neurosurgery, plastic and reconstructive procedures and coronary artery bypass graft ...
Manufactured by:Shockwave Medical Inc. based inSanta Clara, CALIFORNIA (USA)
Neovasc is focused on creating better outcomes for difficult-to-treat cardiology patients through the development of two novel products: Reducer™* for treatment of refractory angina and the Tiara™ System* for treatment of mitral valve regurgitation. Millions of patients worldwide with coronary artery disease who are not candidates for ...
Manufactured by:Baymed Inovative Health Products Inc. based inMerkez/Kilis, TURKEY
Our range includes single drapes and sets developed for cardiothoracic surgery, including heart valve replacements, pacemaker surgery, thoracotomy and coronary artery bypass ...
Manufactured by:P+F Products + Features GMBH based inVienna, AUSTRIA
The EFIL+ Sirolimus Eluting Stent System is a combination product comprised of two regulated components: a device (a coronary stent system) and a drug product (a formulation of Sirolimus contained in a polymer coating) pre mounted on balloon catheter between two platinum iridium radio opaque marker bands. EFIL+ uses a validated formulation of low dose Sirolimus (1.30µg/mm ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
Transmyocardial Revascularization (TMR) is a treatment to help reduce or eliminate chest pain by increasing blood flow to blood deprived areas of the heart muscle for a patient suffering from: Severe Angina, Chronic Angina, Chest Pain (resulting from coronary artery disease). The procedure takes place while the patient is under general anesthesia. When TMR is ...
Manufactured by:Sequana Medical NV based inSint-Denijs Westrem, BELGIUM
The American Heart Association estimates that 6.5 million adults in the United States age 20 and over are affected by heart failure and that number is expected to rise to 8.0 million adults by 2030. Causes of heart failure include coronary artery disease, heart attacks, high blood pressure and faulty heart valves. Heart failure can disturb the normal functioning ...
Manufactured by:Concept Medical Inc. based inTampa, FLORIDA (USA)
Conversion of Sirolimus drug into sub-micron sized particles; Nanocarriers created by encapsulation of sub-micron sized Sirolimus drug into highly biocompatible drug Carrier-phospholipid; Upon inflation of MagicTouch SCB at target site, nanocarriers with Sirolimus drug inside gets transferred to the vessel wall following the principle of co-efficient diffusion; Upon body pH variation, ...
Manufactured by:Sahajanand Medical Technologies Ltd. based inSurat, INDIA
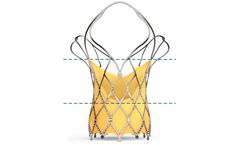
Self-expanding TAVI device to have 2 rows of marker; First Markers are located at Node 1. Second Markers are located at Node 3. First marker helps; In precise implantation of the valve at the targeted implantation zone. To ascertain the depth of implant. Second marker indicates; When the THV leaflets are going to get ...
Manufactured by:Tsunamed, Part of Q3 Medical Group based inWinsen, GERMANY
Offering excellent trackability, pushability, and aspiration volume. The EMAX is a rapid exchange type catheter used for the removal of thrombus and debris in the coronary or peripheral arteries via percutaneous ...