Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Immunoassay Intended Equipment Supplied In Europe
10 equipment items found
Manufactured by:Roche Diagnostics International AG based inRotkreuz, SWITZERLAND
Elecsys® Total-Tau CSF (tTau) is an in vitro diagnostic immunoassay intended for the quantitative determination of the total tau protein concentration in human cerebrospinal fluid ...
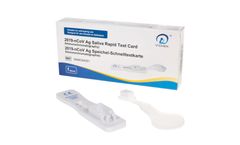
Manufactured by:ulti med Products (Deutschland) GmbH based inAhrensburg, GERMANY
The Test Card is a lateral flow immunoassay intended for the in vitro qualitative detection of N-protein antigen from 2019-nCoV in human saliva specimens. The Test Card can be used for individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection. ...
Manufactured by:Bio-Rad Laboratories, Inc. based inHercules, CALIFORNIA (USA)
The BioPlex 2200 HIV Ag-Ab assay is a multiplex flow immunoassay intended for the simultaneous qualitative detection and differentiation of the individual analytes HIV-1 p24 antigen, HIV-1 (groups M and O) antibodies, and HIV-2 antibodies in human serum or plasma (fresh or frozen K2 EDTA, K3 EDTA, lithium heparin, sodium heparin; fresh citrate). This kit is ...
by:Future Diagnostics based inWijchen, NETHERLANDS
The Future Diagnostics STAT-IntraOperative-Intact-PTH (STAT-IO-I-PTH) Immunoassay kit is intended to be used for in vitro quantitative measurement of intact Parathyroid Hormone (PTH) concentrations in human serum and EDTA plasma. This procedure is recommended as an aid during surgery of hypersecreting parathyroid ...
Manufactured by:bioactiva diagnostica GmbH based inBad Homburg, GERMANY
Intended Use:- The Adenovirus IgG ELISA is intended for the qualitative determination of IgG class antibodies against Adenovirus in human serum or plasma (citrate, heparin). Expiry date:- 10-12 Months. Certification:- CE IVD.Company:- bioactiva diagnostica GmbH. Storage condition:- 2 - 8 °C. Running time:- 105 min. Materials Provided:- Microtiterplate, IgG Sample Dilution Buffer, Stop ...
Manufactured by:bioactiva diagnostica GmbH based inBad Homburg, GERMANY
Intended Use:- The Adenovirus IgM ELISA is intended for the qualitative determination of IgM class antibodies against Adenovirus in human serum or plasma (citrate, heparin). Expiry date:- 10-12 Months. Certification:- CE IVD. Company:- bioactiva diagnostica GmbH. Storage condition:- 2 - 8 °C. Running time:- 105 min. Materials Provided:- Microtiterplate, IgM Sample Dilution Buffer, Stop ...
Manufactured by:Creative Diagnostics based inShirley, NEW YORK (USA)
The ANA Screen assay is a qualitative enzyme immunoassay (EIA) intended to screen for the presence of antinuclear antibodies (ANAs) in human serum or plasma as an aid in the diagnosis of certain systemic rheumatic diseases. ...
Manufactured by:bioactiva diagnostica GmbH based inBad Homburg, GERMANY
Intended Use:- The Chikungunya Virus IgM μ-capture ELISA is intended for the qualitative determination of IgM class antibodies against Chikungunya Virus in human serum or plasma (citrate, heparin). Expiry date:- 10-12 Months. Certification:- CE IVD. Company:- NovaTec Immundiagnostica GmbH. Storage condition:- 2 - 8°C. Materials Provided:- Microtiterplate, Sample Dilution Buffer, Stop ...
Manufactured by:bioactiva diagnostica GmbH based inBad Homburg, GERMANY
Intended Use:- The Chikungunya Virus IgG capture ELISA is intended for the qualitative determination of IgG class antibodies against Chikungunya Virus in human serum or plasma (citrate, heparin). Expiry date:- 10-12 Months. Certification:- CE IVD. Company:- NovaTec Immundiagnostica GmbH. Storage condition:- 2 - 8 °C. Running time:- 165 min. Materials Provided:- Microtiterplate, Sample ...
Manufactured by:Technopath Clinical Diagnostics based inBallina, IRELAND
Multichem IA Speciality Quality Control provides tri-level utility. Its proprietary formulation mimics the performance of patient samples and targets key medical decision levels of BNP, PTH, ACTH, PCT and ...