Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
In Vitro Diagnostic Kit Equipment Supplied In Europe
10 equipment items found
Manufactured by:GENERI BIOTECH s.r.o. based inHradec Králové, CZECH REPUBLIC
Detection method: allelic discrimination, real-time PCR; This in vitro diagnostic kit is intended for detection of mutation 4G/5G in PAI1 gene promotor in human genomic DNA. Detection is based on real-time polymerase chain reaction (qPCR) using fluorescently labelled probes (allelic discrimination). ...
Manufactured by:GENERI BIOTECH s.r.o. based inHradec Králové, CZECH REPUBLIC
This in vitro diagnostic kit is intended for detection of mutation G1691A (Leiden) in coagulation factor V in human genomic DNA. Detection is based on real-time polymerase chain reaction (qPCR) using fluorescently labelled probes (allelic ...
Manufactured by:GENERI BIOTECH s.r.o. based inHradec Králové, CZECH REPUBLIC
Detection method: allelic discrimination, real-time PCR; This in vitro diagnostic kit is intended for detection of mutation G20210A in coagulation factor II in human genomic DNA. Detection is based on real-time polymerase chain reaction (qPCR) using fluorescently labelled probes (allelic ...
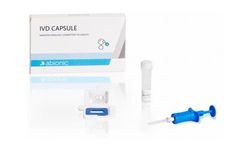
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE Aeroallergens is the first rapid, single-use in vitro diagnostic test for the quantitative determination of circulating immunoglobulin E (IgE) specific to the allergen components Phl p 1 + Phl p 5 (combined), Der p 1 + Der p 2 (combined), Alt a 1, Fel d 1 and Can f 1 in uncoagulated human capillary blood collected from the patient’s ...
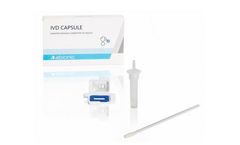
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE COVID-19 is a rapid, single-use in vitro diagnostic test that measures the presence of a specific SARS-CoV-2 viral antigen in saliva. Abionic’s COVID-19 saliva test allows for ultra-fast screening of COVID-19 patients at the point-of-care with lab quality ...
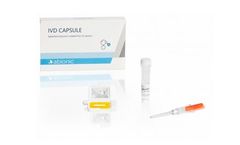
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE Ferritin is a rapid, single-use in vitro diagnostic test for the quantitative measurement of ferritin in capillary and venous whole blood, to aid blood diagnostic testing for iron deficiency in ...
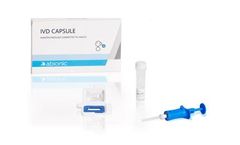
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the determination of Pancreatic Stone Protein (PSP) in blood for the calculation of the cSOFA score at the point of care. The cSOFA score is used to assess the severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in ...
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the quantitative measurement of pancreatic stone protein (PSP) in blood. The test is intended to be used in conjunction with other clinical assessments and laboratory findings to aid in the early detection of nosocomial sepsis in ...
Manufactured by:CLONIT srl based inAbbiategrasso, ITALY
DuplicαRealTime Next Factor V H1299R Kit is an in vitro diagnostic test for the detection of the nucleotide substitution A4070G of the blood coagulation Factor V gene, by Real-Time ...
Manufactured by:Labmaster Ltd. based inKAARINA, FINLAND
The Labmaster LUCIA™ CRP Kit is an in vitro diagnostic solution designed to quantitatively measure C-reactive protein (CRP) from whole blood samples. This near-patient diagnostic tool is intended for use with the semi-automated Labmaster LUCIA™ Analyzer, facilitating healthcare professionals in assessing the body's ...