Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
In Vitro Release Testing Equipment Supplied In Europe
57 equipment items found
Premium
Manufactured by:Bertin Technologies based inMontigny-le-Bretonneux, FRANCE
BEC SARS-CoV-2 RT-LAMP system is designed for the rapid detection of SARS-CoV-2 genome on the field, in less than 30 ...
Manufactured by:ACS Biotech based inVilleurbanne, FRANCE
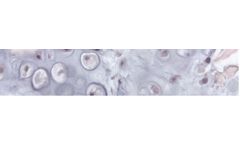
Our strategy to combine the properties of chitosan (natural, biodegradable, biocompatible, cell adhesion) to a 3D hydrogel environment and selected biomolecules has led to develop a matrix (patented) whose performance and biocompatibility have been validated with in vitro tests on human cells in collaboration with the CNRS. ...
Manufactured by:Immatics N.V. based inTuebingen, GERMANY
Immatics’ TCER molecules can be produced and purified utilizing established processes to manufacture ...
Manufactured by:Coris BioConcept based inGembloux, BELGIUM
In vitro rapid diagnostic test for the detection of SARS-CoV-2 antigen in nasopharyngeal secretions. Since many months, several variants did pop-up from countries where the pandemic was particularly active (i.e. UK, Brazil, India and South Africa) and our test has been proven to detect all of ...
Manufactured by:MeKo Manufacturing e.K. based inSarstedt / Hannover, GERMANY
With RESOLOY MeKo offers a bioresorbable magnesium-alloy with extraordinary properties. RESOLOY is an attractive alternative to polymer scaffolds. In comparison to PLLA, it offers about 3 times higher strength, thinner struts, a higher radial force and is applicable for direct stenting. The degradation time of RESOLOY is adjusted by coatings. It has a proven biocompatibility, no shelf life and no ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
Anti-TG is an ELISA-based, automated, in-vitro test system for the quantitative determination of IgG autoantibodies against thyroglobulin (TG) in human serum or plasma. The protein thyroglobulin is responsible for the production and storage of thyroid hormones and is a strong autoantigen. B-lymphocytes with membrane-bound IgM antibodies against thyroglobulin are even detected in the blood of ...
Manufactured by:InActiv Blue bv based inBeernem, BELGIUM
stabilization of RNA/DNA at room temperature, inactivation of pathogens, mucolytic, clinical application: swab, saliva, can also be used as powerful lysis buffer, CE marked IVD. InActiv Blue® is a CE-IVD marked, virus inactivating and RNA stabilizing transport medium for swabs or saliva from patients for in vitro diagnostic testing by RT-qPCR or other molecular method (SARS-CoV-2, influenza ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
The assay contributes to the diagnosis of antiphospholipid syndrome (APS). The clinical symptoms of APS alone are not sufficiently specific to make a definitive diagnosis. Laboratory tests thus play an important role in the diagnosis of the disease. In patients with APS, autoantibodies are formed that bind to phospholipids like cardiolipin or to phospholipid-binding proteins like ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
This assay contributes to the diagnosis of antiphospholipid syndrome (APS). Regardless of their immunoglobulin class, cardiolipin autoantibodies are an important criterion for the diagnosis of APS. Autoantibodies against cardiolipin are also an immunological classification criterion for systemic lupus erythematosus (SLE). In addition, the quantitative determination of anti-cardiolipin is very ...
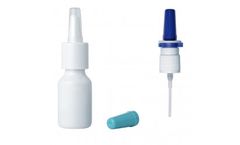
Manufactured by:Nemera based inLa Verpillière, FRANCE
Quality and performance for ENT sprays in regulated markets. The SP270+ and SP370+ are our versatile pumps for both Rx/ OTC drug products and medical devices for ear, nose and throat sprays. They comply with regulated markets requirements. ...
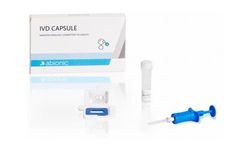
Manufactured by:Abionic SA based inEpalinges, SWITZERLAND
The IVD CAPSULE Aeroallergens is the first rapid, single-use in vitro diagnostic test for the quantitative determination of circulating immunoglobulin E (IgE) specific to the allergen components Phl p 1 + Phl p 5 (combined), Der p 1 + Der p 2 (combined), Alt a 1, Fel d 1 and Can f 1 in uncoagulated human capillary blood collected from the patient’s ...
Manufactured by:Nemera based inLa Verpillière, FRANCE
Off-the-shelf devices available for key identified nasal drugs. Accelerate your sprint to market with our Device Equivalence Program. Based on FDA requirements, we have established a Device Equivalence Program in order to accelerate time-to-market and achieve in-vitro bioequivalence requirements. Our main objective is to preselect and propose an appropriate delivery system per identified drug ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
AMA-M2 is an ELISA-based, automated, in-vitro test system for the quantitative determination of IgG antibodies against mitochondrial M2 subtype antigen in human serum or plasma. Anti-mitochondrial antibodies (AMA) are a heterogeneous group of autoantibodies directed against various proteins of the outer and inner mitochondrial membrane. Autoantibodies of the M2 subtype bind to the E2 subunits of ...
Manufactured by:bioMérieux S.A. based inMarcy l`Etoile, FRANCE
Adenovirus R-gene US Assay is a Real Time PCR in vitro diagnostic test for the rapid and qualitative detection of Adenovirus viral DNA isolated and purified from nasopharyngeal swab or nasopharyngeal wash/aspirate specimens obtained from individuals exhibiting signs and symptoms of acute respiratory ...
Manufactured by:Fujirebio based inMalvern, PENNSYLVANIA (USA)
CanChek Tumor Marker Control Serum is intended for use as an assayed quality control serum to monitor the accuracy of laboratory testing for neuron specific enolase (NSE), S100B protein and squamous cell carcinoma antigen (SCCA). The use of a quality control serum is indicated to verify the performance characteristics of an in vitro diagnostic laboratory test and is an integral part of good ...
Manufactured by:Citeq Biologics based inGroningen, NETHERLANDS
Lepidoglyphus destructor, also known as storage mite, belongs to the Acaridae family. In Europe, Lepidoglyphus destructor has been reported to be a major source of mite allergy in rural and urban environments. Our Lepidoglyphus destructor extract and whole culture are delivered in freeze-dried form and available from ...
Manufactured by:SYMATESE based inCHAPONOST, FRANCE
Absorbable hemostat for haemostatic procedure during surgery with than 800 000 products manufactured per year and CE approval since 2003. Hemostatic pad made from non-denatured, freeze-dried, resorbable native collagen type I, extracted from calf hides. Our absorbable hemostat , preserves its integrity in a damp atmosphere, allowing our hemostatic pad to be handled easily during application. ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
Anti-DGP IgA is an ELISA-based, automated, in-vitro test system for the quantitative determination of IgA antibodies against deamidated gliadin protein epitopes (DGP) in human serum or plasma. These assays are used to support a diagnosis of celiac disease. ...
Manufactured by:Vitrosens Biotechnology A.S. based inÜmraniye, TURKEY
This kit is intended for in vitro quantitative screening of the concentration of Thyroxine (T4) in human serum, plasma, and whole blood samples. Automated and objective results in 15 minutes by immunofluorescent assay. The purpose of this kit is to quantitatively determine the amount of Thyroxine (T4) present in human serum, plasma, and whole blood samples in an in vitro setting. ...
Manufactured by:Sysmex Nederland B.V. based inEtten-Leur, NETHERLANDS
The new standard in routine allergy diagnostics. NOVEOS provides patient comfort with only 4 µl sample volume and performs accurate ...