Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Applications
- 3D bioprinting technology for breast disease
- 3D bioprinting technology for lung disease
- 3D bioprinting technology for brain disease
- 3D bioprinting technology for liver disease
- Diagnosis Solutions for Sepsis
- Solutions for COVID-19 Antigen Saliva Tests
- Rapid Allergy Testing for Allergic Rhinitis & Allergic Asthma Tests
- Screening for Iron Deficiency through Ferritin Blood Levels - Ferritin
- 3D bioprinting technology for skin disease
- 3D bioprinting technology for pancreas disease
- 3D bioprinting technology for prostate disease
- The mat (t) isse bioprothesis: a bio-resorbable tec (tissue engineering chamber)
- Exosomes - Extracellular Vesicles for Validation and Quality Control
- Exosomes - Extracellular Vesicles for Cancer Treatment
- Exosomes - Extracellular Vesicles for Immunomodulating Response
- Exosomes - Extracellular Vesicles for Molecular Therapy
- Exosomes - Extracellular Vesicles for Brain Drug Delivery
- Exosomes - Extracellular Vesicles for Ocular Drug Delivery
Incubator Equipment Supplied In Europe
190 equipment items found
Manufactured by:Tende Electronics Software Engineering Communication Machinery Industry and Trade Ltd. Şti. based inSincan, TURKEY
Tende VAV-TR can be operated by AC network, external DC power supply or by an internal battery. The battery starts to charge when the AC source is connected. The transport incubator can be operated for minimum of two hours with a single battery and minimum of four hours with double ...
Manufactured by:Cobams srl based inSan Lazzaro di Savena, ITALY
The care of ill or premature newborn infants. Technological monitoring and therapy mean special care for ...
Manufactured by:Cobams srl based inSan Lazzaro di Savena, ITALY
The care of ill or premature newborn infants. Technological monitoring and therapy mean special care for ...
Manufactured by:Cobams srl based inSan Lazzaro di Savena, ITALY
The care of ill or premature newborn infants. Transport incubators to move critically sick newborn babies by ambulance or aircraft between ...
Manufactured by:International Biomedical Ltd. based inAustin, TEXAS (USA)
The A750i In-House Transport Incubator is an effective transport solution ideal for moving neonatal patients in and around the hospital. Configurable to a fully loaded NRP compliant transport incubator system, Compact and easy to maneuver around the NICU or L&D Unit, On-board storage of two gas cylinders, Integrated shock absorbers provide a gentle ride ...
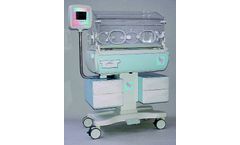
Manufactured by:MEDICOR Zrt based inBudapest, HUNGARY
Main body includes the hood, controller, bassinet, internal battery and observation lamp, skin temperature sensor, IV pole, mattress, adjustable ...
Manufactured by:GINEVRI srl based inAlbano Laziale, ITALY
The BABY SHUTTLE transport incubator is the ideal device for a safety transport in any environmental conditions. By its microprocessor control creates the ideal microclimate for the premature ...
Manufactured by:GINEVRI srl based inAlbano Laziale, ITALY
In the last 60 years GINEVRI has designed and manufactured neonatal incubators which have always been characterized by their original technical solutions and up-to-date performances. ...
Manufactured by:Planer Limited based inMiddlesex, UNITED KINGDOM
The Cellbox incubator is a live cell shipper, designed for the safe transport of oocytes, live cell cultures, tissue and other cell-based samples around the world. Optimal incubation conditions can be maintained throughout the journey, with temperature and CO2 able to be adjusted as required. Cellbox is a portable CO2 incubator suitable for air ...
Manufactured by:Okuman Medikal Sistemler A.Ş based inAnkara, TURKEY
Air and Baby modes with micro-computer controller. AC and DC Power inlet. 12V DC or 24V DC Power inlet for ambulance use. Internal battery capacity ...
Manufactured by:International Biomedical Ltd. based inAustin, TEXAS (USA)
Rugged design for both aeromedical and ambulance transport. Customizable with a wide range of neonatal ventilators and cardiac monitors. Provides dependant and consistent thermoregulation. Compact and lightweight to meet the strict space and weight restrictions of aircraft. Optional Integrated Phototherapy and Pulse Oximetry (Masimo or ...
Manufactured by:Cellbox Solutions GmbH based inNorderstedt, GERMANY
The Cellbox Flight CDI is the only shipping incubator that has been awarded an international flight allowance. Incubation temperatures can be preset between 28°C and 38°C depending on the ambient temperature and a suitable CO2 concentration can be selected between 0% and 18%. TECHNICAL SPECIFICATIONS: The Cellbox Flight CDI, is the first cell shipping CO2 ...
Manufactured by:Cobams srl based inSan Lazzaro di Savena, ITALY
The care of ill or premature newborn infants. Technological monitoring and therapy mean special care for ...
Manufactured by:Tende Electronics Software Engineering Communication Machinery Industry and Trade Ltd. Şti. based inSincan, TURKEY
Tende VAV intensive care incubator is used with a 7-inch color TFT touch screen display. This feature allows easy monitoring and correct entry of operation commands to the incubator. Guiding explanations that pop-up over the screen and animations allow the incubator to be used effectively in a short period of time. ...
Manufactured by:Médipréma Group based inTauxigny, FRANCE
Médipréma’s Nite transport system provides genuine overhead radiant heating which is incorporated inside the incubator canopy, ensuring immediate heat transfer to the infant. The thermal insulation has been carefully designed to protect the baby from environmental disturbances and numerous door openings during transport. The heating system, effective in all ...
Manufactured by:Cellbox Solutions GmbH based inNorderstedt, GERMANY
Dry-ice naturally sublimates into gaseous CO2 – that in turn sustains the passengers in the Cellbox. The source of CO2 does however impact the operation period of the incubator. The Cellbox Ground 2.0 can be operated for more than 72 hours (at 5% CO2), while the Cellbox Flight 2.0 would need a dry ice refill after 32 ...
Manufactured by:Cellbox Solutions GmbH based inNorderstedt, GERMANY
Cellbox Ground CD ushers in a new era of cell shipping: Live Cell Shipping. Incubation temperatures can be preset between 28°C and 38°C depending on the ambient temperature and a suitable CO2 concentration can be selected between 0% and 18%. TECHNICAL SPECIFICATIONS: The Cellbox Ground CD, is the first cell shipping CO2 incubator that provides lab-like ...
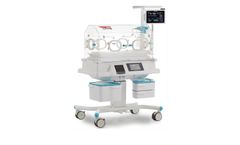
Manufactured by:Médipréma Group based inTauxigny, FRANCE
The Inotherm neonatal intensive care incubator has a precise, reliable and intuitive thermoregulation system with 7 temperature regulation modes. These include the air and skin regulation modes found on all médipréma incubators. In addition, the incubator has a Stop heating mode, which stops the incubator regulating ...
Manufactured by:Oxford Optronix Ltd. based inAbingdon, UNITED KINGDOM
Authentic physoxia in a bench-top hypoxia incubator/workstation. Compact and portable, Substantially redesigned in 2021 for a more spacious working environment., Regulated using oxygen partial pressure for true hypoxia/physoxia, Mimics the in vivo cellular oxygen environment, Rapid equilibration / frugal gas consumption, HEPA filtration built-in, Brilliantly ergonomic design, ...
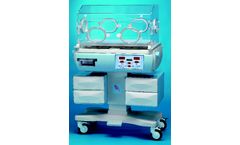
Manufactured by:MEDICOR Zrt based inBudapest, HUNGARY
The functions and operation of BLF-2001 incubators meet the requirements of IEC-601-2-19 product standard and those of the 93/42/EEC Directive. The materials used comply with the hygienic and toxicological requirements and are monitored continuously. The development, production and quality assurance of the incubators are performed in accordance with the ISO 13485 ...