Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Infusion Set Injection Equipment Supplied In Europe
9 equipment items found
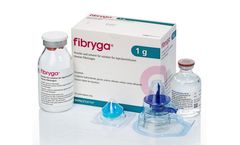
Manufactured by:Octapharma AG based inLachen, SWITZERLAND
Fibryga® is a highly purified and pathogen inactivated human fibrinogen concentrate and an effective and convenient treatment for fibrinogen deficiency. Fibryga offers a number of benefits: concentration is standardised; it is stable at room temperature, can be reconstituted within minutes, and has a low infusion volume; there is no need for thawing or cross-matching blood type and it is ...
Manufactured by:SEQENS Group based inÉcully, FRANCE
Cisatracurium is a nondepolarizing skeletal muscle relaxant for intravenous administration. Cisatracurium acts on cholinergic receptors, blocking neuromuscular transmission. Cisatracurium is one of the ten isomers of the parent molecule, atracurium. Cisatracurium represents approximately 15% of the atracurium ...
Manufactured by:Troge Medical GmbH based inHamburg, GERMANY
TRO-VENOCATH is sterile, non-pyrogenic and atoxic. It shows the following characteristics: - special 3 facet back point bevel of the needle for easy and atraumatic insertion - two movable wings for safe fixation to the skin - Luer Lock cone for immediate sealing of the catheter - FEP or PUR catheter with completely embedded radiopaque stripes - colour coded wings and hub - with injection port for ...
by:Pureims B.V. based inRoden, NETHERLANDS
Amikacin Cyclops™ is an amikacin dry powder inhaler for the treatment of tuberculosis (TB). Inhaled amikacin from the Cyclops™ will result in higher amikacin concentrations in the lungs than conventional infusion or intramuscular injection. This could more rapidly decrease contagiousness by sterilizing the upper airways of patients with ’open’ (smear positive) TB, ...
Distributed by:Mediplast AB based inMalmö, SWEDEN
Different length of tubing between 180-320 cm, Vented or non-vented, PVC-free or PVC DEHP-free material, Roller clamp for maximum precision to ensure consistent and safe infusion rate, Spike without silicone to ensure safe connection to infusion bag/bottle, Liquid filter and air filter, Flowstop with hydrophobic filter which simplifies priming and reduces the risk of leakage and contamination, ...
Manufactured by:ISOJET ÉQUIPEMENTS based inCorbas, FRANCE
The DPE Indus injection machine is designed for applications demanding high precision and control. Utilizing disposable static mixers, it avoids the need for cleaning solvents and ensures accurate dosing. The machine is versatile, being customizable to process a variety of materials including dielectric resins, Polyurethane, Epoxy, or Silicone. Its operation suits multiple techniques such as RTM ...
Manufactured by:Raumedic AG based inDietzenbach, GERMANY
Silicone Injection Molding - Processing of an Outstanding Polymer. Silicone Intelligence Inside: Silicone Intelligence Inside with RAUMEDIC – a real additional benefit connect your ideas with our passion in ...
Manufactured by:Pakumed Medical Products Gmbh based inEssen, GERMANY
TITAN-PORT V are totally implantable port catheter systems for venous implantation. They consist out of a titan chamber (port), a screw closure mechanism, a self-sealing silicone membrane and a catheter. Each system includes a 20 G access needle, a rinsing needle, a vein lifter, the instruction for use, a patient care guide and an implant card. If the port catheter system is a complete set ...
Manufactured by:Pakumed Medical Products Gmbh based inEssen, GERMANY
TITAN-PORTS are totally implantable port catheter systems for venous implantation. They consist out of a titan chamber (port), a screw closure mechanism, a self-sealing silicone membrane and a catheter. Each system includes a 20 G access needle, a rinsing needle, a vein lifter, the instruction for use and an implant card. If the port catheter system is a complete set (recognisable by the suffix ...