Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Medical Device Information Equipment Supplied In Europe
17 equipment items found
Manufactured by:Onda Corporation based inSunnyvale, CALIFORNIA (USA)
The HNR Series needle hydrophones are excellent sensors for laboratory use in high intensity ultrasonic field mapping, with pinpoint access and good spatial resolution. Due to their high sensitivity these hydrophones are commonly operated without ...
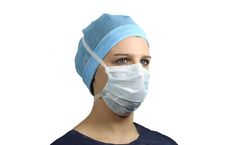
Manufactured by:Kolmi Hopen Medicom Group based inSaint Barthelemy d`Anjou, FRANCE
Features and advantages: Superior comfort thanks to exceptional breathability,All our masks are manufactured in a Clean Room environment ISO 8 (particle and microbiological control), Type IIR protective splash-resistant material (according to ISO 22609:2004),Bacterial filtration efficiency up to 99 %,This product is a CE medical device ...
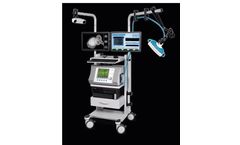
Manufactured by:inomed Medizintechnik GmbH based inEmmendingen, GERMANY
New treatment methods for psychiatry.Non-invasive brain stimulation techniques have been developed to address the large number of patients who may be non-responsive to traditional pharmacological and psychotherapeutic treatment. Therapeutic brain stimulation is particularly relevant for depressive disorders. This is because the treatment can be delivered as necessary to address the frequency and ...
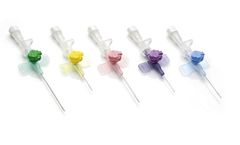
Manufactured by:Troge Medical GmbH based inHamburg, GERMANY
TRO-VENOCATH is sterile, non-pyrogenic and atoxic. It shows the following characteristics: - special 3 facet back point bevel of the needle for easy and atraumatic insertion - two movable wings for safe fixation to the skin - Luer Lock cone for immediate sealing of the catheter - FEP or PUR catheter with completely embedded radiopaque stripes - colour coded wings and hub - with injection port for ...
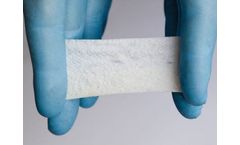
Manufactured by:Kerecis based inArlington, VIRGINIA (USA)
Kerecis Omega3 GraftGuide is not available in all countries and may be known by different names than used on this web-page. Products, product names, indications and claims vary between countries. Not all indications and claims below are for example cleared by the US ...
Manufactured by:Endodiag based inParis, FRANCE
EndoDTectis a blood test based on the analysis of a combination of biomarkers. Changes in their expression levels indicate if a subject has endometriosis or not. Combining high sensitivity and specificity, EndoDTect has the potential to dramatically reduce diagnosis delays. Endodiag is currently conducting validation studies to confirm the predictive value of EndoDTect® for disease ...
Manufactured by:ETKHO Hospital Engineering based inBarcelona, SPAIN
The medical insulation monitor is a device that ensures the integrity of insulation resistance in IT electrical systems, particularly relevant for medical environments. It operates by connecting between the IT circuit and grounding conductor, superimposing a measurement current to monitor insulation resistance levels. In case the resistance falls below a pre-determined threshold, an alarm is ...
Manufactured by:RTI Group AB based inMölndal, SWEDEN
The immaculate new Mako Dental Probe together with the Mako Base Unit offers the ultimate X-ray test tool in Dental applications, with best-in-class accuracy (±1.5 % kV) and advanced detector design. The probe is perfectly designed for CBCT and intraoral applications, in addition to panoramic dental measurements, helpful medical imaging information, featuring a 0.9 mm detector for full ...
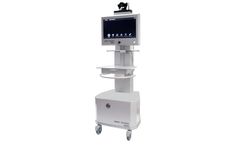
Manufactured by:Meytec GmbH based inWerneuchen, GERMANY
Mobile telemedicine system for medical audio-video communication of the finest class quality. The VIMED TELEDOC HD++ is tailored for the specific needs of the telemedicine applications. It is ergonomically designed fulfilling the different needs of daily medical care. The preferred field of the application is emergency healthcare. The design and construction emphasizes the high stability and ...
Manufactured by:Terragene based inSanta Fe, ARGENTINA
Process Challenge Device for Steam sterilization ...
Manufactured by:Terragene based inSanta Fe, ARGENTINA
Process Challenge Device (KPCD110) for Ethylene Oxide sterilization ...
by:Eizo GmbH based inRülzheim, GERMANY
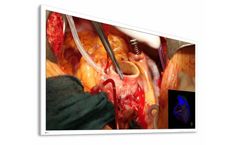
58-inch widescreen surgical monitor with 4K UHD display that faithfully reproduces surgical ...
Manufactured by:Gymna International based inBilzen, BELGIUM
ShockMaster devices are equipped with Gymna’s Guided Therapy System (GTS). GTS software supports and guides the therapist in such manner that focus can be entirely on the ...
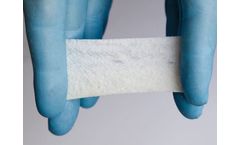
Manufactured by:Kerecis based inArlington, VIRGINIA (USA)
SurgiBind is not available in all countries and may be known by different names than used on this web page. Products, product names, indications and claims vary between countries. Not all indications and claims below are for example cleared by the U.S. ...
Manufactured by:Kerecis based inArlington, VIRGINIA (USA)
Kerecis Omega3 OR is not available in all countries and may be known by different names than used on this web-page. Products, product names, indications and claims vary between countries. Not all indications and claims below are for example cleared by the US ...
Manufactured by:Kerecis based inArlington, VIRGINIA (USA)
Kerecis Omega3 products are not available in all countries and may be known by different names than used on this web-page. Products, product names, indications and claims vary between countries. Not all indications and claims below are for example cleared by the US ...
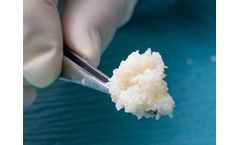
Manufactured by:Kerecis based inArlington, VIRGINIA (USA)
An FDA 510(k) medical device indicated for managing wounds. Not subject to the May 31, 2021, FDA 351 regulation. Kerecis Omega3 MicroGraft is an intact fish-skin graft that has been fragmented into smaller pieces to mold and insert into deep wound spaces and irregular ...