Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Applications
- Innovative disinfection and bio-decontamination technology solutions for healthcare industry
- Innovative disinfection and bio-decontamination technology solutions for Industrial industry
- Innovative disinfection and bio-decontamination technology solutions for hospitality facilities sector
- Innovative disinfection and bio-decontamination technology solutions for transportation sector
- Innovative disinfection and bio-decontamination technology solutions for other business sectors sector
Mri Environments Equipment Supplied In Europe
14 equipment items found
Manufactured by:EKU Elektronik GmbH
based inLeiningen, GERMANY
The maniNO blender for patient supply with a gas blend of oxygen (O2) and nitric oxide (NO) stands out due to easy usability and high NO-precision. It is suited for application in the manual ventilation and can thereby be used in transport as well as in MRI environment. The dosing range of maniNO can be adjusted from 0-40 ppm with an also steplessly adjustable ...
Manufactured by:KOPP Development Inc. based inJensen Beach, FLORIDA (USA)
FerrAlert™ SOLO is Installed in the controlled pre-screening zone. It is the highest sensitivity, full-body patient and personnel scanner available to MRI providers. FerrAlert™ detectors are recognized to be the most accurate ferromagnetic detectors for MRI due to their exclusive location-specificity feature. FerrAlert™ detectors have been ...
Manufactured by:Penlon Limited based inAbingdon, UNITED KINGDOM
The Penlon Prima 451 is a compact MRI anaesthesia system, with advanced patient support modes, and has been designed for use with 1.5 and 3 tesla scanners. Approved for use with 1.5 and 3 tesla scanners, up to 1000 gauss¹. Electronic touch screen ventilator. Designed specifically for use in an MRI environment. ...
Manufactured by:Schiller AG based inBaar, SWITZERLAND
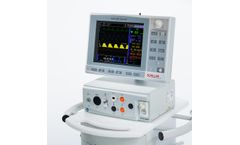
The MAGLIFE Serenity guarantees highest ECG quality during magnetic resonance imaging (MRI) scanning – even under strongest gradient influence. It monitors all vital parameters during anaesthesia in an MRI environment and is specifically designed for adults, children and neonates. ...
Manufactured by:KOPP Development Inc. based inJensen Beach, FLORIDA (USA)
FerrAlert™ Halo II Plus guards at the entrance into the MRI exam room, providing the most precise and reliable detection of medium to large ferromagnetic threats. FerrAlert™ Halo II Plus provides significant assistance in the prevention of serious injury to patients and staff or equipment damage. FerrAlert™ detectors are recognized to be the most accurate ...
Manufactured by:Bentley InnoMed GmbH based inHechingen, GERMANY
The BeSmooth Peripheral Stent System is indicated for the treatment of de novo or restenotic artherosclerotic lesions in protected peripheral ...
by:CyNexo Srl based inUdinese, ITALY
Spir-0 is an innovative subject breathing activity monitoring tool, extremely compact and simple to use. Its operating principle is based on a fiber optic sensor which makes it compatible with use in MRI, fMRI and EEG environments and applications. Spir-0 is able to accurately measure the respiratory behavior of a subject by detecting small variations in the air ...
Manufactured by:Bentley InnoMed GmbH based inHechingen, GERMANY
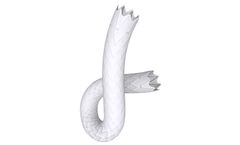
This peripheral stent graft system excels with a high radial force whilst maintaining extraordinary flexibility and a low profile. The cobalt-chrome stent platform is covered with a micro-porous ePTFE ...
Manufactured by:Bentley InnoMed GmbH based inHechingen, GERMANY
This peripheral stent graft uniquely combines an extraordinary radial force with a remarkable ...
Manufactured by:Bentley InnoMed GmbH based inHechingen, GERMANY
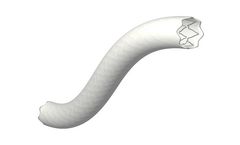
One-layer peripheral stent graft system for large diameter vessels. The stent platform made of cobalt-chrome is covered with a micro-porous ePTFE membrane and is characterized with a high radial force at predictable foreshortening. The BeGraft Aortic Stent Graft System is not available in the ...
Manufactured by:99 Technologies S.A. based inLugano, SWITZERLAND
At 99T we know that prevention via efficacious disinfection must be applied widely and persistently to be effective. So, we have made a deliberate effort make the use of our disinfection technology as widely adoptable as possible with the 99MB Waves-Shielder. Surface’s morphology of Magnetic Resonance Imaging (MRI) devices makes the disinfection process difficult and time consuming. Hence, ...
Manufactured by:Bentley InnoMed GmbH based inHechingen, GERMANY
This extraordinary product combines a highly flexible one-layer stent graft with a low-profile balloon catheter to offer an unrivalled small guiding catheter compatibility. The cobalt-chrome stent platform is covered with a micro-porous ePTFE membrane. The BeGraft Coronary Stent System is not for available in the ...
by:Advanced Healthcare Technology Ltd. (AHT) based inHerts., UNITED KINGDOM
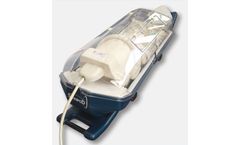
Its non-invasive nature is especially important considering the size and nature of the patients requiring treatment and early diagnosis critical to direct treatment efficiently and at the earliest opportunity. The Scan Pod is designed to provide a warm and comfortable environment for the infant patient, in which to undergo MRI, CT or X-Ray imaging. ...
Manufactured by:Biotronik SE & Co. KG based inBerlin, GERMANY
For many years, patients with a pacemaker, ICD or CRT device, have been denied access to MRI. The cardiac devices were not approved for MRI scanning and the influence of magnetic resonance imaging on implanted cardiac systems was not yet ...