Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Myeloma Equipment Supplied In Europe
18 equipment items found
Manufactured by:Creative Bioarray based inShirley, NEW YORK (USA)
The NB-4 cell line was derived from the marrow of a patient with acute promyelocytic leukemia (APL; M3 in the FAB nomenclature) in second relapse in ...
Manufactured by:Celyad Oncology based inMont-Saint-Guibert, BELGIUM
CYAD-211 is a short hairpin RNA (shRNA)-based allogeneic CAR T candidate for the treatment of relapsed or refractory multiple myeloma (r/r MM). CYAD-211 is engineered to co-express a B-cell maturation antigen (BCMA) chimeric antigen receptor and a single shRNA hairpin which interferes with the expression of the CD3ζ component of the TCR complex. CYAD-211 is currently being ...
Manufactured by:Takeda Pharmaceutical Company Limited based inChuo-ku, JAPAN
Bortezomib (brand name: Velcade) is a treatment for multiple myeloma (a cancer of the plasma cells) and mantle cell lymphoma (a cancer of the lymph ...
by:Genmab A/S based inCopenhagen V, DENMARK
Daratumumab is a human IgG1k monoclonal antibody (mAb) that binds with high affinity to the CD38 molecule, which is highly expressed on the surface of multiple myeloma cells.1 Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license to develop, manufacture and commercialize daratumumab from Genmab. A subcutaneous formulation of daratumumab, ...
Manufactured by:LAVA Therapeutics N.V. based inUtrecht, NETHERLANDS
Our lead program, LAVA-051, is a unique, humanized gamma-delta bsTCE targeting CD1d-expressing tumors, including multiple myeloma, chronic lymphocytic leukemia (CLL), and acute myeloid leukemia (AML). We have achieved preclinical proof-of-concept with LAVA-051 by demonstrating efficacy and safety in a variety of preclinical models. LAVA-051 uniquely activates both ...
Manufactured by:SYnAbs S.A. based inGosselies, BELGIUM
That's why we've created SynAbs in the first place, based upon an exclusive asset: the rat-LOU species and rat-LOU IR983 myeloma cell line, developed in 1983 by Hervé Bazin in Catholique University of Louvain-la-Neuve (UCL). ...
Manufactured by:The Menarini Group based inFlorence, ITALY
NEXPOVIO is a first-in-class, oral exportin 1 (XPO1) inhibitor. XPO1 is a nuclear exporter protein that transport from the nucleus to the cytoplasm several proteins, including tumor suppressors, oncogenes, and proteins involved in governing cell growth; it is often overexpressed, and its function mis-regulated in several types of cancers. By inhibiting the XPO1 protein, tumor suppressors proteins ...
by:Biorbyt Ltd based inCambridge, UNITED KINGDOM
Monoclonal antibodies; one of the most widely used tools in biological research, with the potential soon to become important therapeutics, have been with us for just over 40 years. These antibodies are monovalent, bind to a single epitope and are produced by a single B-lymphocyte clone. The first monoclonal antibody was generated in mice in 1975 by Milstein and Kohler, using the hybridoma ...
Manufactured by:Oxford Gene Technology IP Limited based inOxford, UNITED KINGDOM
The P53 probe mix consists of a 161kb probe, labelled in red that covers the whole P53 (TP53) gene and the flanking regions. The probe mix also contains a control probe for the 17 centromere (D17Z1) that is labelled in ...
Manufactured by:Teknimed based inL’union, FRANCE
OPACITY+® is a self-curing and ready to use low viscosity bone cement with a very high content of radiopaque agent for a perfect and safety visualization. Its composition has been optimized to get the most suitable handling properties for vertebroplasty & kyphoplasty. ...
Manufactured by:Heraeus Medical GmbH based inWehrheim, GERMANY
OSTEOPAL V is a specialty low-viscosity bone cement utilized predominantly for spinal surgeries such as percutaneous vertebroplasty and balloon kyphoplasty. The cement is notable for its high radiopacity due to its high content of zirconium dioxide, an X-ray contrast agent, which greatly improves visibility under imaging techniques. Additionally, the cement is uniquely colored with a ...
Manufactured by:Oxford Gene Technology IP Limited based inOxford, UNITED KINGDOM
The CKS1B/CDKN2C product consists of a 182kb probe, labelled in red, covering the entire CKS1B gene and flanking regions, including the PYGO2 and ZBTB7B genes, and a green probe covering a 170kb region, including the entire CDKN2C gene, the D1S1661 marker and the centromeric end of the FAF1 ...
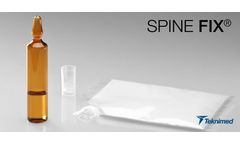
Manufactured by:Teknimed based inL’union, FRANCE
SPINE FIX® is a ready to use bone cement with a high amount of radiopaque agent for vertebroplasty, kyphoplasty & pedicular screw injection procedures. SPINE FIX® allows an excellent consolidation of the vertebral boby with an effective and rapid pain relief. The handling times of SPINE FIX® have been specifically developed to give to the surgeon a comfortable application ...
Manufactured by:Heraeus Medical GmbH based inWehrheim, GERMANY
OSTEPAL Plus is a specialized low-viscosity bone cement designed for use in spinal surgeries, particularly in procedures such as vertebroplasty and kyphoplasty aimed at augmenting and stabilizing vertebral bodies. This product features a low-viscosity formulation which allows for prolonged working times, providing surgeons with the flexibility needed during complex procedures. OSTEOPAL Plus is ...
Manufactured by:Teknimed based inL’union, FRANCE
F20® radiopaque bone cement is a ready to use, medium viscosity, low exothermic, self-curing bone cement with a high content of radiopaque agent for a perfect level of visualization and safety required during percutaneous vertebral bone augmentation procedures. Its composition has been optimized to get the most suitable handling properties for surgeon’s convenience. F20® comes as an ...
by:Stemline Therapeutics, Inc. based inNew York, NEW YORK (USA)
CD123 is expressed on multiple malignancies including BPDCN, AML, certain myeloproliferative neoplasms (MPNs), myelodysplastic syndrome (MDS), chronic myeloid leukemia (CML), B-cell acute lymphoid leukemia (B-ALL), hairy cell leukemia, and Hodgkin’s and certain non-Hodgkin’s lymphomas. In addition to expression on tumor bulk, CD123 expression has been reported on the cancer stem cells ...
by:Biorbyt Ltd based inCambridge, UNITED KINGDOM
Plasma cells are specialised B lymphocytes that secrete antibodies (glycoproteins) in response to infection by antigens (toxins or foreign substances). Antibodies, also known as immunoglobulins (Ig), usually have a high affinity to a specific antigen, binding only to one epitope/binding site on an antigen. Monoclonal (Mab) or polyclonal (pAb) antibodies are used in scientific research for a wide ...
by:Stemline Therapeutics, Inc. based inNew York, NEW YORK (USA)
XPO1 has been shown to regulate nuclear export of many of the major tumor suppressor proteins and oncogenic cell growth regulators. Overexpression of XPO1 has been reported in many cancer types and is associated with aggressive tumor behavior and poor patient prognosis. Inhibition of XPO1 has been shown to restore tumor suppressor function and proper cell cycle regulation, leading to apoptosis of ...