Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Newborn Screening Equipment Supplied In Europe
8 equipment items found
Manufactured by:ZenTech s.a. based inLiège, BELGIUM
The Immunomat™ is intended for neonatal screening laboratories performing between 400 and 1000 daily determinations. With a maximum capacity of 7 microplates, it optimizes the rate of the laboratory by allowing the processing of different parameters in parallel. The handling of this robust instrument is easy and the software is intuitive to use. The Immunomat greatly facilitates and ...
Manufactured by:ZenTech s.a. based inLiège, BELGIUM
The NEONATAL TOTAL-T4 Screening ELISA kit is an ELISA-type quantitative test used to quantify the totality of thyroxine (T4) contained in a test dried blood samples on 903 or 226 blotting paper. This test is for congenital hypothyroidism newborn ...
Manufactured by:ZenTech s.a. based inLiège, BELGIUM
The test only identifies SMA homozygotes. This qualitative test is dedicated only to spinal muscular atrophy (SMA) newborn screening and is performed on a dried blood sample on 903® or 226 blotted paper. Parameter ; Non-mutated SMN1 allele. ...
Manufactured by:ViennaLab Diagnostics GmbH based inVienna, AUSTRIA
Congenital adrenal hyperplasia (CAH) is an inherited disorder affecting steroid hormone synthesis. The CAH StripAssay® detects the most common point mutations, whereas the CAH RealFast™ CNV Assay identifies copy number variations (CNVs) in the CYP21A2 gene in patients with CAH. For comprehensive genetic analysis both assays should be used in ...
Manufactured by:Yourgene Health based inManchester, UNITED KINGDOM
The Yourgene Cystic Fibrosis Base assay has receieved IVDR certification! A product with IVDR certification enables clinician and patient confidence in a higher quality test - where accuracy ...
Manufactured by:MAICO Diagnostics GmbH based inBerlin, GERMANY
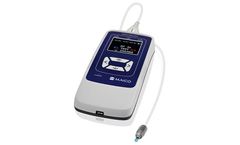
OAE Testing with Screening or Diagnostic Functions. Our ERO SCAN offers frequency specific TEOAE and/or DPOAE evaluation with easy handling. The sharp organic LED display presents line and bar diagrams for easy, direct evaluation. Choose between ERO•SCAN with screening or diagnostic ...
Manufactured by:MAICO Diagnostics GmbH based inBerlin, GERMANY
Our easyScreen unites ABR, TEOAE and DPOAE screening capabilities in one device to meet your need for a cost efficient 2-step ABR/OAE Hearing Screener.easyScreen will lighten your daily workload in many ways: The device fits right into your pocket and helps you save time with its binauralautomated ABR screening to test both ears at the same time. The integrated CE-Chirp® stimulus generates ...
Manufactured by:Medic Labor s.r.o. based inBratislava, SLOVAKIA
Dedicated random access prenatal screening platform from the leader in the field. DELFIA® Xpress has been developed to streamline workflows in laboratories and clinics providing prenatal screening services. Already in use in more than 50 countries, DELFIA Xpress offers a range of benefits critical for operational efficiency., The speed and flexibility of random access, The simplicity and ease ...