Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Ophthalmology Solution Equipment Supplied In Europe
8 equipment items found
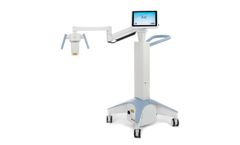
Manufactured by:Gkteso GmbH based inBobingen, GERMANY
The Microsurgical Bed for OEMs is a high-precision, fully motorized surgical platform engineered for complex procedures in ophthalmology, urology, neurosurgery, and microsurgical disciplines. It offers maximum stability, ergonomic access, and smooth, vibration-free motion where accuracy during surgery is key. With motorized height, tilt, and rotation control, it enables exact patient ...
Manufactured by:Gkteso GmbH based inBobingen, GERMANY
The Ophthalmology Eye Surgery Bed is a precision-engineered patient positioning system specifically developed for ophthalmic laser procedures such as LASIK, PRK, and SMILE. With ultra-smooth, vibration-free motion control and joystick-controlled fine adjustment (0.02 mm (20 microns) lateral/longitudinal; 0.05 mm vertical), it enables millimeter-accurate alignment while maximizing patient ...
Manufactured by:AneticAid Ltd. based inBaildon, UNITED KINGDOM
Outstanding surgical access - exceptional patient comfort; Outstanding surgical access with exceptional patient comfort - Compact, light weight and supremely manoeuvrable – the complete solution for ophthalmology. Ideal for patient transport, intubation, theatre and recovery, the QA3™ Ophthalmic has been designed with the patient and the clinician in ...
Manufactured by:Farmigea S.p.A. based inPisa, ITALY
Intensive lubrication and protection of the ocular surface. HydraMed Forte lubricates and protects the ocular surface, and provides long-lasting relief. HydraMed Forte is an ophthalmic solution with TS-Polysaccharide and high-molecular weight Sodium Hyaluronate. The concentration of the mixture is calibrated in such a way as to ensu- re the lubrication even in cases of more intense ...
Manufactured by:ViGeneron GmbH based inPlanegg, GERMANY
vgAAV, ViGeneron’s proprietary AAV Vector platform, is our next generation vector platform based on novel engineered AAV capsids. In a unique in vivo directed-evolution approach we generated our vgAAV vectors. The vgAAV vector platform enables a superior transduction of target cells and is designed to efficiently cross biological barriers. These attributes allow vgAAV vectors to target a ...
Manufactured by:Farmigea S.p.A. based inPisa, ITALY
Moisturising, lubricant and normalising ophthalmic solution. HydraMed is an ophthalmic solution with TS-Polysaccharide (TSP®) and high-molecular weight Sodium Hyaluronate, obtained by biotechnological synthesis. The combination of these two substances gives a synergic normalizing action on the corneo-conjunctival surface, and protects from irritation due to environmental elements (wind, ...
Manufactured by:Medicem Group a.s. based inPraha 10, CZECH REPUBLIC
MEDICEM focuses on R&D for ophthalmic surgery solutions featuring its proprietary, versatile WIGEL® bioanalogic hydrogel material platform. WIGEL® is a transparent hydrogel with stable 3D structure, improved biocompatibility, and a clinically proven safety and efficacy profile in intraocular use. It offers high efficacy of modification by femtosecond laser, enabling controlled ...
Manufactured by:Glaukos Corporation based inSan Clemente, CALIFORNIA (USA)
iLink is the first and only FDA-approved corneal cross-linking procedure that slows or halts the progression of keratoconus and helps preserve vision. Watch this video for unique patient and doctor perspectives about progressive keratoconus and learn how the condition is treated with iLink™ corneal cross-linking. ...