Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Real Time Micro Pcr Equipment Supplied In Europe
125 equipment items found
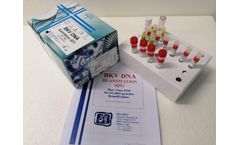
Manufactured by:Dia.Pro Diagnostic Bioprobes s.r.l based inSesto San Giovanni, ITALY
The BKV DNA Quantitation Real-Time PCR kitcoded BKVDNAQT.CE is intended for the quantitative detection of the BK Virus DNA in human plasma and urine with a simultaneous control of the amplification/extraction reaction through an Internal Control (IC). The kit has been adapted for the use on the Real-Time Thermacyclers ABI 7500 Sequence Detection System® (Software SDS version 1.3.1, ...
by:TECOmedical AG based inSissach, SWITZERLAND
Detection of terbinafine resistances in native patient material. The test detects resistance-causing mutations on the squalene epoxidase gene, and additionally relevant Trichophyton ...
Manufactured by:BioVendor – Laboratorni Medicina a.s. based inBrno, CZECH REPUBLIC
Type: Two-Tailed RT/ PCR Primers. Applications: COVID-19. Shipping: Frozen. Upon receipt, store the product at the temperature recommended below. Storage/Expiration: Store the kit at -20°C. Under these conditions, assay components are stable till the expiry date is over. (See the expiry date indicated on the kit ...
by:TECOmedical AG based inSissach, SWITZERLAND
High sensitive PCR assays for the detection of viral, bacterial, fungal and parasital infections using monoplex and mutiplex test ...
Manufactured by:BioVendor – Laboratorni Medicina a.s. based inBrno, CZECH REPUBLIC
Type: Two-Tailed RT/ PCR Primers. Applications: COVID-19. Shipping: Frozen. Upon receipt, store the product at the temperature recommended below. Storage/Expiration: Store the kit at -20°C. Under these conditions, assay components are stable till the expiry date is over. (See the expiry date indicated on the kit ...
Manufactured by:altona Diagnostics GmbH based inHamburg, GERMANY
All RealStar PCR reagents are designed for quantification/qualititative detection and/or differentiation of pathogen-specific RNA/DNA in human matrix/matrices. A heterologous amplification system (Internal Control) is included to identify possible PCR inhibition. Please note the regulatory status of our three different product groups ...
by:TECOmedical AG based inSissach, SWITZERLAND
Using a patented 10-minute extraction step, the DermaGenius 2.0 multiplex RT- PCR detects more than 95% of the clinically important Dermatophytes in nail, skin and hair samples within 3 ...
Manufactured by:BIORON Diagnostics GmbH based inRömerberg, GERMANY
BIORON’s RealLine Cyclers for any kind of Real-Time-PCR-Assays are available with 48 well and 96 well blocks for research labs or high sample throughput applications in diagnostic ...
Manufactured by:BIORON Diagnostics GmbH based inRömerberg, GERMANY
BIORON’s RealLine Cyclers for any kind of Real-Time-PCR-Assays are available with 48 well and 96 well blocks for research labs or high sample throughput applications in diagnostic ...
Manufactured by:BioGX, Inc. based inBirmingham, ALABAMA (USA)
BioGX real-time PCR-based detection reagents are manufactured and packaged as open system reagents (OSR) for use with open system platforms and have to be validated by the user. Examples of open system platforms are the BD MAX™ System, Applied Biosystems QuantStudio™ 5, and Bio-Rad CFX96 Touch™ real-time PCR platforms. Research use only reagents are not intended for human or ...
Manufactured by:BioGX, Inc. based inBirmingham, ALABAMA (USA)
BioGX real-time PCR-based detection reagents are manufactured and packaged as open system reagents (OSR) for use with open system platforms and have to be validated by the user. Examples of open system platforms are the BD MAX™ System, Applied Biosystems 7500 Fast Dx, QuantStudio™ 5, Bio-Rad CFX96 Touch™ and Bio-Rad CFX384 Touch™real-time PCR platforms. Research use only ...
Manufactured by:Anatolia Geneworks based inSultanbeyli – Istanbul, TURKEY
It is well known that the accuracy of the thermal cycles and achieving temperature uniformity between tubes, so that all samples undergo the same thermal conditions, are prerequisite for a reliable PCR reaction. Key to a precise and sensitive quantification analysis lies in the error-free detection and analysis of low levels of fluorescence. In order to realize these fundamentals, Montania® ...
Manufactured by:BioGX, Inc. based inBirmingham, ALABAMA (USA)
BioGX real-time PCR-based detection reagents are manufactured and packaged as open system reagents (OSR) for use with open system platforms and have to be validated by the user. Examples of open system platforms are the BD MAX™ System, Applied Biosystems QuantStudio™ 5, and Bio-Rad CFX96 Touch™ real-time PCR platforms. Research use only reagents are not intended for human or ...
Manufactured by:Dia.Pro Diagnostic Bioprobes s.r.l based inSesto San Giovanni, ITALY
The Enterovirus RNA Real-Time PCR kit coded ENTERORNA.CE is intended for the qualitative detection of Enterovirus RNA in human plasma and CSF samples (Cerebral Spinal Fluid) with a simultaneous control of the amplification reaction through an Internal Control (IC). ...
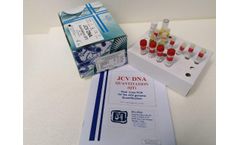
Manufactured by:Dia.Pro Diagnostic Bioprobes s.r.l based inSesto San Giovanni, ITALY
The JCV DNA Quantitation Real-Time PCR kit coded JCVDNAQT.CE is intended for the quantitative detection of the JC Virus DNA in human plasma ,urine and cerebrospinal fluid (CSF) with a simultaneous control of the amplification/extraction reaction through an Internal Control (IC). ...
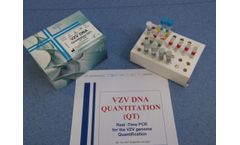
Manufactured by:Dia.Pro Diagnostic Bioprobes s.r.l based inSesto San Giovanni, ITALY
The VZV DNA Quantitation (QT) Real-Time PCR kit coded VZVDNAQT.CE is intended for the quantitative detection of Varicella-Zoster virus DNA in human plasma and CSF (cerebrospinal fluid) with a simultaneous control of the extraction/amplification reaction through an Internal Control (IC). ...
Manufactured by:altona Diagnostics GmbH based inHamburg, GERMANY
The AltoStar PCR kits are based on real-time PCR technology, utilizing polymerase chain reaction (PCR) for amplification of specified target sequences and target-specific probes in order to detect amplified DNA or ...
Manufactured by:AB ANALITICA s.r.l. based inPADUA, ITALY
The REALQUALITY RQ-CMV STANDARD is a CE-IVD diagnostic kit containing known quantified standards for the quantification of Cytomegalovirus (CMV) by Real Time PCR. This product has to be used in combination with the REALQUALITY RQ-CMV kit. ...
Manufactured by:FRIZ Biochem GmbH based inNeuried, GERMANY
COVID-19 direct RT-PCR Omicron is designed to detect SARS-CoV-2 RNA and the COVID-19 Omicron variant (Y505H mutation) in parallel directly from (an) unprocessed patient sample(s) on a commercial qPCR cycler. ...
Manufactured by:BioGX, Inc. based inBirmingham, ALABAMA (USA)
BioGX real-time PCR-based detection reagents are manufactured and packaged as open system reagents (OSR) for use with open system platforms and have to be validated by the user. Examples of open system platforms are the BD MAX™ System, Applied Biosystems QuantStudio™ 5, and Bio-Rad CFX96 Touch™ real-time PCR platforms. Research use only reagents are not intended for human or ...