Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Transcatheter Device Equipment Supplied In Europe
6 equipment items found
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
In the early days of transcatheter mitral valve repair, the goal was a successful procedure that reduced mitral regurgitation (MR) to less than 3+; residual MR grade 2+ was considered acceptable.1,2 Compelling new data on more than 5400 patients show that residual MR grades 0–1+ are significantly associated with superior patient outcomes when compared with residual MR 2+, including ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
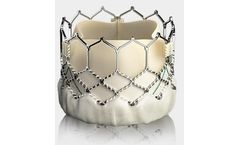
Built on the proven SAPIEN platform, the SAPIEN 3 Ultra transcatheter heart valve was designed with patient needs in ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
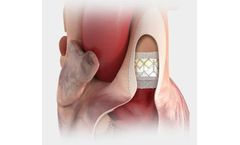
Designed for predictable deployment. The SAPIEN XT valve may be used in the aortic position via a broad range of access routes - transfemoral, transapical and ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Builds upon the proven strengths of the SAPIEN valve with 5 years of durability. The SAPIEN 3 valve is the only valve approved for valve-in-valve procedures in both the aortic and mitral positions, allowing patients at high or greater surgical risk to avoid an additional open heart ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
The first transcatheter heart valve approved for pre-stented transannular patches. Also approved for use in the pulmonic position in conduits and ...
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
PTFE is a well-established biomaterial with a long clinical history of implantation. It has been approved by regulatory bodies for several decades and is used extensively in a range of vascular ...