Show results for
Refine by
Bio Pharma Equipment Supplied In France
12 equipment items found
Manufactured by:ASEPTIC GROUP based inLimonest, FRANCE
The focus of this text is on manifolds and molded assemblies made from custom biopharmaceutical-grade silicone or thermoplastic elastomers (TPE). These products are designed to minimize and reduce the requirement for fittings, connections, or assembly in creating a complete aseptic transport system. The lack of entrapment and leak-point areas in these solutions ensures superior product integrity. ...
Manufactured by:MGA Technologies based inCivrieux d’Azergues, FRANCE
The ergonomic stainless steel pharmaceutical trolley by MGA Technologies is a versatile mobile workstation tailored for the intricate demands of the medical and biopharmaceutical industries. Constructed from durable stainless steel, it is designed to meet cGMP standards and is CE certified, ensuring both safety and compliance in sensitive environments. The trolley is highly adaptable, featuring ...
Manufactured by:ASEPTIC GROUP based inLimonest, FRANCE
Sani-Tech STHT-C tubing is specially designed for the biopharmaceutical sector, offering ultra-pure tubing tailored for high purity applications. Fabricated from platinum-cured silicone, it demonstrates resistance to extreme temperatures, ozone, and chemical attacks, ensuring durability and dependability in demanding environments. Its material composition allows it to withstand repeated ...
Manufactured by:Transgene based inIllkirch-Graffenstaden, FRANCE
A new generation of multifunctional oncolytic virus, TG6002 has been designed to combine the mechanism of oncolysis (targeted destruction of the cancer cell) with the production of chemotherapy (5-FU), directly into the ...
Manufactured by:Mega Biopharma based inCrosne, FRANCE
Product / use: biological cements, watertight cements, for fixing the implant in place in the bone. Key properties: osteogenic, homeostatic, biocompatible, bioabsorbable, radiopaque, no volume reduction, no exothermic reaction. ...
Manufactured by:Mega Biopharma based inCrosne, FRANCE
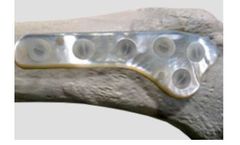
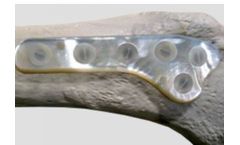
Product / use: plates, screws, rods, spacers, intramedullary nails. Key properties: osteogenic, homeostatic, biocompatible, bioerodible, radiopaque, no volume reduction, no exothermic reaction. ...
Manufactured by:Veracyte based inMarseille Cedex 9, FRANCE
Veracyte offers a unique multiplex tissue imaging and analysis approach combining multiple technologies perfectly adapted to the comprehensive characterization of the tumor microenvironment. ...
Manufactured by:Mega Biopharma based inCrosne, FRANCE
Product / Use: injectable bone substitute for vertebroplasty and kyphoplasty. Key properties: osteogenic, homeostatic, biocompatible, bioabsorbable, radiopaque, no volume reduction, no exothermic reaction. ...
Manufactured by:System-c Bioprocess based inSaint-Paul Trois Châteaux, FRANCE
The PAK System is an advanced platform specifically designed for the continuous purification of biopharmaceuticals, such as monoclonal antibodies. This single-use platform performs four unit operations: diafiltration, chromatography, viral inactivation, and inline concentration. It automates flow and mass rates for purifying up to 500 L of cell culture daily. The system utilizes four control ...
Manufactured by:Angany Inc. based inSt-Jean, QUEBEC (CANADA)
We aim to provide safe, dependable and lasting relief from allergy. We have developed a unique therapeutic approach based on a proprietary allergy vaccine technology to address the specific challenge of allergy (eBioparticle-Potentiated ...
Manufactured by:Mega Biopharma based inCrosne, FRANCE
Bone grafts, remedying loss of bone substance, bone reconstruction and bone fusion are being performed increasingly frequently in orthopaedic and traumatic surgery (vertebral fusion, vertebroplasty, joint reconstruction, traumatology, etc.). As a result, there is an increasing need for bone fillers. The ageing population and obesity are found to be the main reasons. Since the early 90’s, ...
Manufactured by:Thermo Fisher Scientific based inWaltham, MASSACHUSETTS (USA)
MAM has been broadly embraced by the biotherapeutic industry and regulatory leaders in recent years. Major biopharmaceutical companies are making significant investments to establish a MAM workflow to future proof the development and manufacturing of their biotherapeutics. Historically, the implementation of MAM has not been easy or fast due to the absence of a complete commercial MAM solution. ...