Refine by
Medical Sterilized Equipment Supplied In France
18 equipment items found
Manufactured by:All-Wrap 4 SS Packaging Machinery based inVerdun, FRANCE
The machine VPM 300 produces peelable packs with one medical sterilization rolls on paper/film, Tyvek®/ film, or other complexes as foil /foil … The material feeding station and the pre-cutting station allowing the introduction of the product, as well as that of the welding / cross-section are controlled by three servomotors. ...
Manufactured by:France Chirurgie Instrumentation S.A.S. (FCI) based inPARIS, FRANCE
FCI punctal plugs are preloaded on a disposable inserter for a progressive release & an easy ...
Manufactured by:France Chirurgie Instrumentation S.A.S. (FCI) based inPARIS, FRANCE
FCI Painless Plug is the ultimate result of our extensive research on dry eye syndrome for the last 30 years. It relieves the discomfort associated with dry eye syndrome simply, quickly, effectively & durably and greatly improves the patients quality of ...
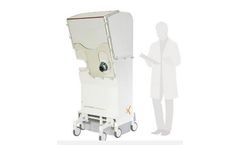
Manufactured by:MDS Medical Devices Sterilizers based inIvry le Temple, FRANCE
Worldwide preferred technology for low temperature sterilization of single use medical devices is Ethylen Oxide (EtO). This sterilization process is optimized by MDS to offer fully turnkey solution for medical device manufacturers and sterilization services providers. Our sterilization cycle is ...
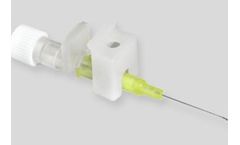
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
IV cannulas with wing holder / cover, specially designed for one-handed insertion. IV cannulas with wing holder / cover, specially designed for one-handed insertion. The device carries small wings, specially designed for easy gripping and safe clamping to allow secure fixation to ...
Manufactured by:Sterimed based inCoulommiers, FRANCE
Ethypel Performance SP 63 gsm is a medical cellulose-based overall coated top web solution designed to provide a controlled and consistent sealing performance for optimized peel ability requirements when combined to films made of a PE seal layer (PA/PE ; PP/PE ; PET/PE, etc.). ...
Manufactured by:OPIA Technologies based inPARIS, FRANCE
TEARPRIM is a sterile medical device designed to sample the tear film. It enables further analysis of tear components to help diagnose several ocular surface disorders, such as dry eye or allergies. The TEARPRIM device was developed in close collaboration with Quinze-Vingts Hospital and ...
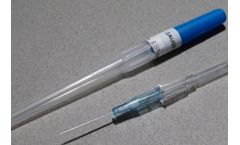
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
JCM MED Intravenous Cannulas / Catheters allow the introduction or withdrawal of fluids from the human circulatory system. The short flexible and kink-resistant catheter is introduced into a blood vessel over a hollow introducer needle. Pen-type IV cannulas offer the advantage of potentially fully enclosing the used needle for safe disposal. They are also fitted with a hydrophobic filter. ...
Manufactured by:OPIA Technologies based inPARIS, FRANCE
EYEPRIM is a sterile medical device designed to perform conjunctival impressions of the living eye. It enables further analysis of cells and biomarkers to help diagnose several ocular surface disorders, such as dry eye, allergies or infections. The EYEPRIM device is based on an original idea, suggested by Christophe Baudouin, MD, PhD, Chairman of Department of ...
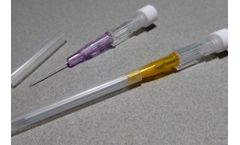
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
JCM MED Intravenous Cannulas / Catheters allow the introduction or withdrawal of fluids from the human circulatory system. The short flexible and kink-resistant catheter is introduced into a blood vessel over a hollow introducer needle. These IV cannulas are fitted with an injection port that allows the supplementation of a drug into the cannula itself by a syringe without a needle, and prevents ...
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
JCM MED Intravenous Cannulas / Catheters allow the introduction or withdrawal of fluids from the human circulatory system. The short flexible and kink-resistant catheter is introduced into a blood vessel over a hollow introducer needle. Also called « one-way » IV cannulas, these cannulas may be supplied, in their sophiticated version, with hydrophobic filters to avoid blood spillage ...
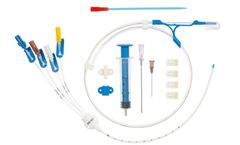
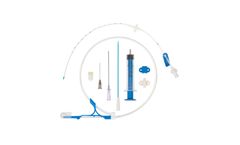
Manufactured by:Prodimed based inNeuilly en Thelle, FRANCE
Central venous catheters for introduction by the Seldinger technique. Class III sterile medical ...
Manufactured by:JCM MED based inSuze-la-Rousse, FRANCE
JCM MED Intravenous Cannulas / Catheters allow the introduction or withdrawal of fluids from the human circulatory system. The short flexible and kink-resistant catheter is introduced into a blood vessel over a hollow introducer needle. IV cannulas with wings only are fitted with flexible wings for easy and proper fixation. These IV cannulas may be supplied with an additional injection port / ...
Manufactured by:Prodimed based inNeuilly en Thelle, FRANCE
Central venous catheters for introduction by the Seldinger technique. Class III sterile medical ...
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
Maintain a needlefree closed system to help minimize exposure to hazardous drugs and comply with USP. Prohibits the escape of hazardous drug or vapor concentrations, Blocks the transfer of environmental contaminants into the system, Eliminates needlestick injuries while minimizing , hazardous drug exposure. With the ChemoLock needlefree CSTD, it has never been easier to keep yourself safe from ...
Manufactured by:Matachana Group based inBarcelona, SPAIN
The Series S100 steam sterilizers incorporate in its technical design the latest advances in safety and ...
Manufactured by:SYMATESE based inCHAPONOST, FRANCE
NEVELIA® dermal regeneration template is used to reconstruct the dermis following a skin loss. NEVELIA® dermal regeneration template is a sterile medical device consisting of a collagen layer to promote dermal regeneration and a reinforced silicone layer acting as a pseudo-epidermis. ...
Manufactured by:Lemer Pax based inChapelle-Sur-Erdre Cedex, FRANCE
The model Cathpax AF Adjustable offers an optimal 2mm lead equivalence radiation protection to the operators during cardiac electrophysiology procedures, while adapting to the occupational habits. It is adapted to all EP procedures: explorations, AF ablations, flutter, supraventricular tachycardia etc…and is totally complementary to 3D navigation systems. Cathpax AF Adjustable is the most ...