Show results for
Refine by
Cardiac Procedures Equipment Supplied In Germany
16 equipment items found
Manufactured by:Dispomedica - A Neuromedex brand based inHamburg, GERMANY
The sternum wires are used for sternum closure in intrathoracic surgery. The atraumatic, bend-resistant needles with a specialised and precise needle-cut, allow easy penetration of the ...
Manufactured by:AS Medizintechnik GmbH based inTuttlingen, GERMANY
Micro needle holders, scissors and forceps are required for modern and state of the art micro disciplines. Neuro-, hand-, plastic-, vascular-, cardiac- and ENT Surgery do set very high demands on surgeons and also on their handheld micro ...
Manufactured by:Dispomedica - A Neuromedex brand based inHamburg, GERMANY
Temporary Myocardial Pacing Electrodes are the most common type of pacing wires, placed directly at the epicardium in selectable ways to match procedural requirements individually. Our Unipolar Electrodes offer an extensive stimulation area and requires placement of a minimum of two electrodes for pacing. ...
Manufactured by:KLS Martin Group based inTuttlingen, GERMANY
Reusable? Indeed. Our reusable aortic punch is the only one of its kind in the world, and quite rightly so. Our SolidBlack coating guarantees long and sharp use. The adjustable cleaning position is unique, leaving no room for ...
Manufactured by:KLS Martin Group based inTuttlingen, GERMANY
In minimally invasive cardiac surgery, every movement of the hand must be right. All the steps of the surgical procedure are planned meticulously and carried out with utmost precision and care. The patient and his/her safety are the focus of every operation. Our marCore® instruments are intended to help you achieve the best possible results in your efforts to ...
Manufactured by:ERGOSURG GmbH based inIsmaning, GERMANY
Cardiac Navigation System catheter-based navigation for cardiac surgery. The ERGOSURG Cardiac Navigation System supports the surgeons’ spatial understanding during catheter-based minimally invasive cardiac surgeries and is based on a tracked navigation mandrin inserted into supported standard catheters or a direct optical tracking of supported standard guidewires/catheters. It visualizes ...
Manufactured by:KLS Martin Group based inTuttlingen, GERMANY
The new marTract retractor system for minimally invasive coronary artery bypass (MIDCAB) includes two individually adjustable traction devices. For sufficient stability, a large amount of force is no longer required when pulling the rib arches and the system is easily ...
Manufactured by:International Group medical technology and electronics GmbH based inBremen, GERMANY
Our MAX Series HF generators have the functions of bloodless cutting, electrocauterization, electrocoagulation and high efficient electrocoagulation. It comes with automatic compensation function, memory setting and pad testing. All our devices can be used in different type of operations such as: General surgery, Cardiac surgery, Gynaecology, Othopaedics, Neurosurgery, Microsurgery and more. ...
Manufactured by:Baymed Inovative Health Products Inc. based inMerkez/Kilis, TURKEY
Baymed® disposable cardiovascular drapes provide the strength, versatility and efficiency needed for the critical demands of cardiovascular surgery. Baymed® disposable cardiovascular drapes, made with heavy-duty fabric and Advanced Bayteks® Reinforcement fabric for fluid management in fenestrations, may eliminate the need for costly drape layering. Baymed® disposable drapes for ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
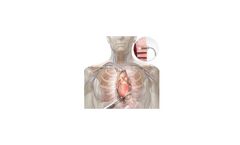
Transmyocardial Revascularization (TMR) is a treatment to help reduce or eliminate chest pain by increasing blood flow to blood deprived areas of the heart muscle for a patient suffering from: Severe Angina, Chronic Angina, Chest Pain (resulting from coronary artery disease). The procedure takes place while the patient is under general anesthesia. When TMR is used as a sole therapy, the surgeon ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
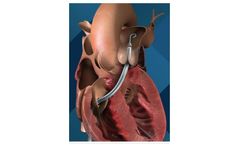
The Impella RP heart pump provides temporary, circulatory support for patients who develop right heart failure. It is the only FDA-approved heart pump indicated for patients experiencing acute right heart failure or decompensation following left ventricular assist device implantation, myocardial infraction, heart transplant or open-heart ...
Manufactured by:Pfm Medical Hico Gmbh based inKoln, GERMANY
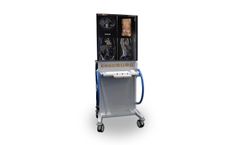
HICO-VARIOTHERM 555 is the allrounder among the hypo-/hyperthermia systems and appreciated for its reliable warming and cooling of patients in intensive care units and operating rooms. Featuring a water temperature range of 5 to 40 °C, this system covers all areas of ...
Manufactured by:CytoSorbents Corporation based inMonmouth Junction, NEW JERSEY (USA)
he CytoSorb therapy* is based on an extracorporeal blood purification procedure that was shown to effectively reduce excessive levels of inflammatory mediators (see “The Adsorber”). This is intended to alleviate the excess systemic inflammatory response (“cytokine storm”) associated with systemic hyperinflammation or septic shock. Thus, the life-threatening complications ...
Manufactured by:W.O.M. World of Medicine GmbH based inBerlin, GERMANY
In Minimally Invasive Medicine, good visibility and precise incisions are the nuts and bolts. However, both are only possible when there is enough space for the scope and medical instruments. Insufflators and Pumps are needed to dilate body cavities reliably with medical CO2 or fluid for stable internal surgical sites. WOM is the global leader and pioneer for Insufflators in the field of ...
Manufactured by:Biotronik SE & Co. KG based inBerlin, GERMANY
For many years, patients with a pacemaker, ICD or CRT device, have been denied access to MRI. The cardiac devices were not approved for MRI scanning and the influence of magnetic resonance imaging on implanted cardiac systems was not yet ...
Manufactured by:EKU Elektronik GmbH
based inLeiningen, GERMANY
In June 2010 EKU Elektronik received the outpout orientated innovation award "Success" by the Investitions- und Strukturbank Rheinland-Pfalz (ISB) GmbH for the successful development of the NO-application device NO-A. EKU Elektronik received the award for the NO-A, a therapy device for the application of nitric oxide (NO) therapy gas in the ventilation flow of an intensive care patient in ...