Show results for
Refine by
Cardiac Version Minimct Patient Equipment Supplied In Germany
5 equipment items found
Manufactured by:GETEMED Medizin- und Informationstechnik AG based inTeltow, GERMANY
Using the PhysioMem PM 100, a patient suffering from intermittent cardiac arrhythmias can record a 2-lead, 40 second ECG anytime and anywhere. Either at regular intervals or if the patient experiences cardiac related symptoms, he or she simply presses the four electrodes attached to the back of the device firmly onto the chest and presses the start button. Once the recording has finished, it is ...
Manufactured by:GANSHORN Medizin Electronic GmbH based inNiederlauer, GERMANY
Whether for differential diagnosis or for medically validated care of athletes: Spiro-ergometry has become an indispensable procedure for cardio-pulmonary function diagnostics. The GANSHORN PowerCube Ergo System provides detailed information for diagnosis, therapy or training support. The pneumotachograph with variable aperture is lightweight and insensitive to wetness. The fast, highly stable ...
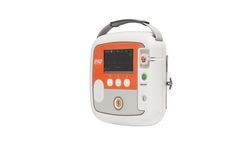
Manufactured by:CU Medical Systems, Inc. based inWonju-Si, SOUTH KOREA
This device is a semi-automated external defibrillator that can be administered on sudden cardiac arrest (SCA) patients. CU-SP2 is an easy-to-use Semi-Automated External Defibrillator (AED) that is small, light, and portable, and uses a battery. The AED automatically or manually reads the sudden cardiac arrest (SCA) patient’s electrocardiogram (ECG) and determines if a cardiac arrest that ...
Manufactured by:CytoSorbents Corporation based inMonmouth Junction, NEW JERSEY (USA)
he CytoSorb therapy* is based on an extracorporeal blood purification procedure that was shown to effectively reduce excessive levels of inflammatory mediators (see “The Adsorber”). This is intended to alleviate the excess systemic inflammatory response (“cytokine storm”) associated with systemic hyperinflammation or septic shock. Thus, the life-threatening complications ...
Manufactured by:Biotronik SE & Co. KG based inBerlin, GERMANY
For many years, patients with a pacemaker, ICD or CRT device, have been denied access to MRI. The cardiac devices were not approved for MRI scanning and the influence of magnetic resonance imaging on implanted cardiac systems was not yet ...