Refine by
Fibula Equipment Supplied In Germany
6 equipment items found
Manufactured by:aap Implantate AG based inBerlin, GERMANY
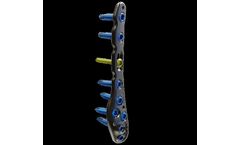
The set comprises LOQTEQ® distal tibia plates for medial and anterolateral application as well as lateral distal fibula plates. Number and placement of plate holes as well as the screw trajectories are designed for treatment of a wide range of fracture types. The distal design respects the minimal soft tissue coverage and helps preventing potential irritation due to ...
Manufactured by:Dieter Marquardt Medizintechnik GmbH based inSpaichingen, GERMANY
Especially in geriatric traumatology and with compromised soft tissue, wound healing becomes the focus of therapy. The VITUS-Fi Fibula Nail System was developed to treat unstable ankle fractures intramedullary. Small incisions and a stable fixation have the aim of shorten the immobilization phase of the patients as much as ...
Manufactured by:Dieter Marquardt Medizintechnik GmbH based inSpaichingen, GERMANY
The WINSTA-FiT plating system by Marquardt Medizintechnik offers anatomically shaped plates that have been specially developed for locking plate fixation of fractures of the distal fibula and tibia. Focusing on the essentials, a clearly arranged instrumentation and full compatibility with the Marquardt small fragment platform characterize the ...
Manufactured by:AF Medical GmbH based inSpaichingen, GERMANY
The XLOC 3.5 instrument set is for the application of the XLOC plates & screws for the upper and lower extremities. It includes instruments specifically designed for the XLOC implants as well as instrumentation for the conventional 3.5 cortical and 4.0 cancellous ...
Manufactured by:AF Medical GmbH based inSpaichingen, GERMANY
The XLOC plate set can be customized according to your needs. Following XLOC plates can be equipped in this ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
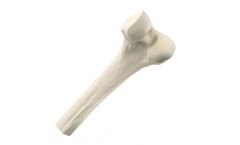
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their final packaging ...