Refine by
Venous Blood Sample Equipment Supplied In Germany
6 equipment items found
Manufactured by:KABE LABORTECHNIK GmbH based inNümbrecht-Elsenroth, GERMANY
Treated test tubes for reticulocyte count from venous or capillary blood in a ratio of 1:1 or 2:1; Test tubes with 100 µl staining solution for reticulocyte count from venous or capillary blood. The sample material is added to the staining solution in the desired ratio, mixed gently, and after 20 - 30 ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
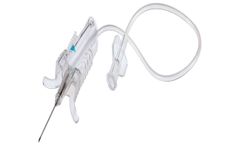
Sample with Confidence: A used needle can cause a needlestick injury, even if it is only exposed momentarily before being shielded. This can cause anxiety for healthcare professionals and patients alike. By utilising a user-centric design that provides protection once the needle is removed from the vein, Unistik® ShieldLock allows HCPs to draw large volumes of blood with ease and helps to ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
Sample with Confidence: A used needle may cause a needlestick injury, even if it is only exposed momentarily before being shielded. This may cause anxiety for healthcare professionals and patients alike. With its one-handed, in-vein activated safety shield, Unistik® ShieldLock Ultra helps to minimise the risk of needlestick injuries and give peace of mind for ...
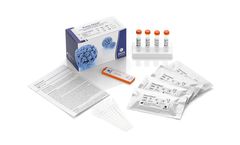
Manufactured by:Abviris Deutschland GmbH based inAhrensburg, GERMANY
Innovative solutions for the serological detection of HPV16 induced cancer. From a diagnostics point of view it is irrelevant to detect the very common HPV infections but rather important to identify HPV induced deseases. The presence of increased HPV16 L1 antibodies is a highly specific indicator for altered cells in patients which have not been vaccinated against HPV. An elevated level of these ...
Manufactured by:Pakumed Medical Products Gmbh based inEssen, GERMANY
TITAN-PORT V are totally implantable port catheter systems for venous implantation. They consist out of a titan chamber (port), a screw closure mechanism, a self-sealing silicone membrane and a catheter. Each system includes a 20 G access needle, a rinsing needle, a vein lifter, the instruction for use, a patient care guide and an implant card. If the port catheter system is a complete set ...
Manufactured by:Pakumed Medical Products Gmbh based inEssen, GERMANY
TITAN-PORTS are totally implantable port catheter systems for venous implantation. They consist out of a titan chamber (port), a screw closure mechanism, a self-sealing silicone membrane and a catheter. Each system includes a 20 G access needle, a rinsing needle, a vein lifter, the instruction for use and an implant card. If the port catheter system is a complete set (recognisable by the suffix ...