Refine by
Venous Sample Equipment Supplied In Germany
5 equipment items found
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
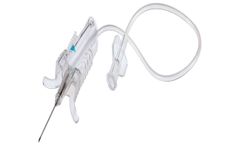
Sample with Confidence: A used needle can cause a needlestick injury, even if it is only exposed momentarily before being shielded. This can cause anxiety for healthcare professionals and patients alike. By utilising a user-centric design that provides protection once the needle is removed from the vein, Unistik® ShieldLock allows HCPs to draw large volumes of blood with ease and helps to ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
Sample with Confidence: A used needle may cause a needlestick injury, even if it is only exposed momentarily before being shielded. This may cause anxiety for healthcare professionals and patients alike. With its one-handed, in-vein activated safety shield, Unistik® ShieldLock Ultra helps to minimise the risk of needlestick injuries and give peace of mind for ...
Manufactured by:Abviris Deutschland GmbH based inAhrensburg, GERMANY
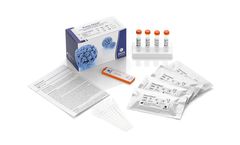
Innovative solutions for the serological detection of HPV16 induced cancer. From a diagnostics point of view it is irrelevant to detect the very common HPV infections but rather important to identify HPV induced deseases. The presence of increased HPV16 L1 antibodies is a highly specific indicator for altered cells in patients which have not been vaccinated against HPV. An elevated level of these ...
Manufactured by:Pakumed Medical Products Gmbh based inEssen, GERMANY
TITAN-PORT V are totally implantable port catheter systems for venous implantation. They consist out of a titan chamber (port), a screw closure mechanism, a self-sealing silicone membrane and a catheter. Each system includes a 20 G access needle, a rinsing needle, a vein lifter, the instruction for use, a patient care guide and an implant card. If the port catheter system is a complete set ...
Manufactured by:Pakumed Medical Products Gmbh based inEssen, GERMANY
TITAN-PORTS are totally implantable port catheter systems for venous implantation. They consist out of a titan chamber (port), a screw closure mechanism, a self-sealing silicone membrane and a catheter. Each system includes a 20 G access needle, a rinsing needle, a vein lifter, the instruction for use and an implant card. If the port catheter system is a complete set (recognisable by the suffix ...