Refine by
Tissue Damage Equipment Supplied In Ireland
5 equipment items found
Manufactured by:Aran Biomedical Teoranta based inSpiddal, IRELAND
The principle benefit of a lubricious coating is to substantially reduce surface friction, facilitating implant delivery and deployment, while additional benefits may be recognised in-vivo, such as reduced potential for tissue damage, lower bacterial adherence or biofilm formation. ...
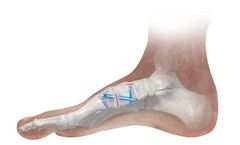
Manufactured by:Paragon 28, Inc. based inEnglewood, COLORADO (USA)
The Phantom® Intramedullary Nail is the first Paragon 28® intramedullary nail and offers a unique option for correcting hallux valgus at the first TMT joint. The nail and threaded peg construct is zero profile and minimizes the soft tissue disruption of the first metatarsal and medial cuneiform during a Lapidus procedure. The Phantom® Intramedullary Nail instrumentation system ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc is designed for use using a lateral traspsoas or retroperitoneal (i.e., anterolateral) surgical approach to access disc space. Access through the annulus can be achieved using the PerQdisc™ Disc Access System (Figure 3). Once annular access is achieved, a nuclectomy (removal of nucleus material) is performed through the disc access using various tools to remove most of the ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc Implant Delivery Device (Figure 4) is then inserted through the Access Cannula into the enucleated disc space. Contrast is injected into the inner chamber of the implant. This aids in visualization as well as provides real-time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a curable, polymer containing barium sulfate. The ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The SST PerQdisc Nucleus Replacement System is intended to replace the nucleus pulposus of the intervertebral disc in the L1-S1 spinal region in patients with single-level discogenic pain. The patient may have single or multi-level degenerative disc changes but the discogenic pain must be limited to a single level. The patient must be skeletally mature, at least 21 years of age, presenting with ...