- Home
- Equipment
- latin america
- reconstructive and regenerative surgery
Refine by
Locations
- Latin America
- Antigua & Barbuda
- Argentina
- Aruba
- Bahamas
- Barbados
- Belize
- Bolivia
- Brazil
- Cayman Islands
- Chile
- Colombia
- Costa Rica
- Cuba
- Dominica
- Dominican Republic
- Ecuador
- El Salvador
- Grenada
- Guatemala
- Guyana
- Haiti
- Honduras
- Jamaica
- Mexico
- Netherlands Antilles
- Nicaragua
- Panama
- Paraguay
- Peru
- Puerto Rico
- St. Kitts & Nevis
- St. Lucia
- St. Vincent & The Grenadines
- Suriname
- Trinidad
- Uruguay
- Venezuela
Reconstructive And Regenerative Surgery Equipment Supplied In Latin America
5 equipment items found
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Fortiva Porcine Dermis is a non-crosslinked acellular porcine dermal matrix offering a safe and natural biologic option. Fortiva implants are processed through the Tutoplast® Tissue Sterilization Process and terminally sterilized via validated low-dose gamma irradiation to achieve a sterility assurance level (SAL) of 10-6. Fortiva Porcine Dermis is a ready-to-use biologic implant that acts as ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Cortiva Allograft Dermis is a non-crosslinked acellular dermal matrix offering a safe and natural biologic option and sterilized to a sterility assurance level (SAL) of 10-6 through the Tutoplast® Tissue Sterilization ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
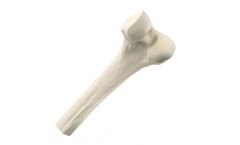
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their final packaging ...
Manufactured by:Vital Sutures based inFort Myers, FLORIDA (USA)
Polydioxanone is a monofilament, synthetic absorbable suture that meets the USP requirements, except for the diameter, which is ruled by the British Pharmacopeia. It is degraded by non-enzymatic hydrolysis. It has a minimum absorption process after 90 days of being implanted, and the thread is completely reabsorbed within 6 months after implantation. This suture has a high tensile strength. ...
Manufactured by:Hyperbaric SAC based inLima, PERU
Hyperbaric oxygen therapy (HBOT) has become a specialized modern medical intervention with a history dating back to 1662 when British Doctor Henshaw hypothesized about the benefits of increased atmospheric pressure. By the 19th century, practitioners in Europe and North America were creating hyperbaric chambers to treat various medical conditions. Today, HBOT is widely used worldwide, facilitated ...