- Home
- Equipment
- netherlands
- clinical trial
Show results for
Refine by
Clinical Trial Equipment Supplied In Netherlands
17 equipment items found
Manufactured by:Lifetech Scientific (Shenzhen) Co., Ltd. based inShenzhen, CHINA
Nitinol wire frame coated with Titanium Nitride (TiN). Decrease the dissolution of nickel ion efficiently, expected safe long-term biocompatibility. Promote the growth of endothelial tissue, lessen thrombus complication. Superior superelastic, effectively reduce atrioventricular block ...
Manufactured by:Capricorn Life Sciences b.v. based inThe Hague, NETHERLANDS
ARTERINORM capsules is a food supplement containing a complex of natural substances which are beneficial for the physiological control of cholesterol plasma levels. The efficacy of this product is documented in several published clinical ...
Manufactured by:Caelus Health based inAmsterdam, NETHERLANDS
E. hallii: as basis of a drug to treat Type 2 diabetes has already been tested in 3 clinical trials in MetS/prediabetes and the next trial in Type 2 diabetes is scheduled to start in the first half of ...
Manufactured by:InSilicoTrials Technologies based inStafford, VIRGINIA (USA)
PCa GnRH Agonists Simulator enables simulations of clinical trials on a virtual population of prostate cancer patients being treated with a GnRH agonist ...
Manufactured by:InSilicoTrials Technologies based inStafford, VIRGINIA (USA)
A tool that performs in silico clinical trials to assess the neutropenic effects of a chemotherapeutic agent in a virtual population of cancer patients ...
Manufactured by:Capricorn Life Sciences b.v. based inThe Hague, NETHERLANDS
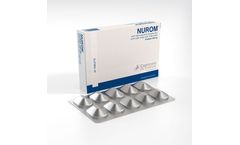
NUROM bi-layer controlled release tablets is a combination of natural elements, based on Alpha-Lipoic acid, Carnosine, Zinc and Vitamin B with clinical trials showing efficacy in the prevention and treatment of (diabetic) neuropathy. NUROM can also be used in Peripheral Neuropathies due to compression, trauma, inflammation, resulting from the use of other drugs, ...
Manufactured by:Kiadis Pharma NV based inAmsterdam, NETHERLANDS
K-NK002: A phase 2 clinical study evaluating haplo-identical NK cells to prevent post-transplant relapse in patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). This study was designed based on promising clinical proof-of-concept data from MD Anderson Cancer Center. The phase 2 trial for K-NK002 will be conducted in ...
Manufactured by:ViciniVax BV based inGroningen, NETHERLANDS
Our first product candidate Vvax001, a therapeutic cancer vaccine against (pre)malignant cervical lesions, is currently in clinical development. We outsource process development and GMP manufacturing to experienced CMO’s, leading to a first clinical trial, which has been started in ...
Manufactured by:LAVA Therapeutics N.V. based inUtrecht, NETHERLANDS
LAVA-051 uniquely activates both Vγ9Vδ2 T cells as well as invariant natural killer T cells, which represent another conserved immune effector cell population, in a target dependent manner. We plan to begin a Phase I/IIa clinical trial in patients with relapsed and/or refractory multiple myeloma and CLL in the first half of ...
Manufactured by:Kiadis Pharma NV based inAmsterdam, NETHERLANDS
We believe this mechanism-of-action means Amlitelimab could be applicable to a range of autoimmune and inflammatory diseases. In our Phase 1 clinical trial in healthy volunteers, Amlitelimab was able to block T cell-driven skin inflammation while being well tolerated. We believe the immune-modulating mechanism of Amlitelimab has broad potential therapeutic ...
Manufactured by:Alnylam Pharmaceuticals, Inc. based inCambridge, MASSACHUSETTS (USA)
The first and only FDA-approved prescription medication for the treatment of primary hyperoxaluria type 1 (PH1). OXLUMO is a prescription medicine for the treatment of PH1 to lower oxalate in urine in children and ...
by:XELTIS BV based inEindhoven, NETHERLANDS
Fully synthetic restorative pulmonary heart valve for Right Ventricular Outflow Tract reconstruction, overtime turning into a living heart valve made of patient’s own ...
Manufactured by:Kiadis Pharma NV based inAmsterdam, NETHERLANDS
Alomfilimab SAR445256 (formerly KY1044) is a human monoclonal IgG1 that selectively binds to Inducible T cell CO-stimulator (ICOS), a protein expressed at high levels on immunosuppressive regulatory T cells and at lower levels on effector T cells. Alomfilimab is designed to exert anti-tumour activity through preferential depletion of intra-tumoral regulatory T cells and stimulation (agonism) of ...
by:Pureims B.V. based inRoden, NETHERLANDS
Amikacin Cyclops™ is an amikacin dry powder inhaler for the treatment of tuberculosis (TB). Inhaled amikacin from the Cyclops™ will result in higher amikacin concentrations in the lungs than conventional infusion or intramuscular injection. This could more rapidly decrease contagiousness by sterilizing the upper airways of patients with ’open’ (smear positive) TB, ...
by:veriNOS pharmaceuticals GmbH based in, NETHERLANDS
Ronopterin is an allosteric inhibitor of inducible NO synthase (iNOS) that rapidly decreases the excessive production of NO resulting from acute overactivation of this enzyme. Ronopterin has no significant effect on the physiological production of NO by other constitutive NO-synthesizing enzymes that are essential for brain and body ...
Manufactured by:Active Implants LLC based inMemphis, TENNESSEE (USA)
The NUsurface® Meniscus Implant is a meniscus replacement in patients with persistent knee pain following medial meniscus surgery. Meniscus replacement has the potential to fill the gap between minimally invasive meniscus repair and total knee replacement. The implant is made from polycarbonate-urethane (PCU) – a medical grade plastic. As a result of its unique materials and composite ...
Manufactured by:DSM based inHeerlen, NETHERLANDS
Discover our implantable drug delivery systems made of bioresorbable polymers that release precise quantities of therapeutic agents gradually over time. DSM is a global leader in biomedical materials science – experience that we are now using to create advanced sustained release drug products as implantable or injectable delivery forms. DSM offers two proprietary, highly versatile polymer ...