- Home
- north america
- Equipment
Refine by
Biomedical Medical Equipment Supplied In North America
28 equipment items found
Manufactured by:Bezwada Biomedical LLC based inHillsborough, NEW JERSEY (USA)
Synthetic absorbable polymers derived from natural amino acids have excellent engineering properties, tunable hydrolysis profiles, and are able to release safe and biocompatible molecules and amino acids upon absorption at the site of action in a controlled manner. We are pleased to offer a portfolio of amino acids functionalized with safe and biocompatible molecules such as lactic and glycolic ...
Manufactured by:Ivy Biomedical Systems, Inc based inBranford, CONNECTICUT (USA)
590494 - (Bag of 40). 590494-CS – (Case of 600). ...
Manufactured by:Getein Biotechnology Co.,Ltd. based inNanjing, CHINA
Can be used as a aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection, it should not be used to diagnose acute SARS-CoV-2 infection. ...
Manufactured by:Bezwada Biomedical LLC based inHillsborough, NEW JERSEY (USA)
At Bezwada Biomedical, we are pleased to offer a portfolio of hydrolysable amines for a variety of pharmaceutical and biomedical applications. Our expanded portfolio consists of numerous revolutionary linker and crosslinker amines derived from safe and biocompatible molecules including amino acids, lactic acid and glycolic ...
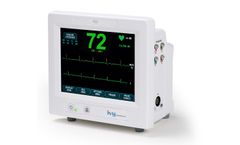
Manufactured by:Ivy Biomedical Systems, Inc based inBranford, CONNECTICUT (USA)
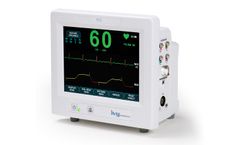
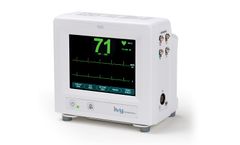
The Model 7810 is Ivy Biomedical System’s newest product introduction providing dual (cardiac and respiratory) gating functionality used in applications requiring precision ECG R-wave and respiratory cycle synchronization for gating host systems on and off. The Model 7810 incorporates an intuitive, menu-driven, touch-screen user interface, simplifying operation for clinicians and ...
Manufactured by:Ivy Biomedical Systems, Inc based inBranford, CONNECTICUT (USA)
The Model 7600 is Ivy Biomedical System’s 5th generation cardiac trigger monitor used in applications requiring precision ECG R-wave synchronization for gating host systems on and off. The Model 7600 incorporates an intuitive, menu-driven, touch-screen user interface, simplifying operation for clinicians and ...
Manufactured by:Ivy Biomedical Systems, Inc based inBranford, CONNECTICUT (USA)
The PAM Module is an embedded instrumentation pack which provides an accurate gating trigger for use in an MRI environment. The PAM-200 is a complete solution consisting of ECG Acquisition, an Optical Plethysmograph for pulse acquisition, and a pneumatic respiration pad for respiration ...
Manufactured by:Getein Biotechnology Co.,Ltd. based inNanjing, CHINA
Getein CK-MB Fast Test Kit is used as an aid in the clinical diagnosis, prognosis and evaluation of myocardial injury such as Acute Myocardial Infarction (AMI), Unstable Angina, Acute Myocarditis and Acute Coronary Syndrome ...
Manufactured by:Getein Biotechnology Co.,Ltd. based inNanjing, CHINA
Getein CK-MB/cTnI/Myo Fast Test Kit is used as an aid in the clinical diagnosis, prognosis and evaluation of myocardial injury such as Acute Myocardial Infarction (AMI), Unstable Angina, Acute Myocarditis and Acute Coronary Syndrome ...
Manufactured by:Getein Biotechnology Co.,Ltd. based inNanjing, CHINA
Getein CK-MB/cTnI/H-FABP Fast Test Kit is used as an aid in the clinical diagnosis, prognosis and evaluation of myocardial injury such as Acute Myocardial Infarction (AMI), Unstable Angina, Acute Myocarditis and Acute Coronary Syndrome ...
Manufactured by:Bezwada Biomedical LLC based inHillsborough, NEW JERSEY (USA)
Imagine polyurethanes that are completely hydrolysable, biocompatible and have tunable physical and mechanical properties similar to those of commercially available medical grade biostable polyurethanes. At Bezwada Biomedical, we not only imagined it, we have made it into a reality. We are pleased to offer a portfolio of absorbable polyurethanes and their precursors for technical evaluation and ...
Manufactured by:Bezwada Biomedical LLC based inHillsborough, NEW JERSEY (USA)
Polyoxaester development is based on aliphatic diacids possessing oxygen atoms adjacent to their α-carbons. These diacids are then polymerized in conjunction with a diol by means of a polycondensation reaction. Since organic chemists have used the term ‘oxa’ to designate the replacement of an ethylene group by an oxygen atom, these diacids have been called oxadiacids, and the ...
Manufactured by:IC Biomedical, LLC based inCartersville, GEORGIA (US) (USA)
Available on Models XT20, XT 21 and XT34, this optional accessory provides a visual and audible alarm when the liquid nitrogen level drops below a preset level – usually 25 percent of the container’s capacity. CLICK HERE for more ...
Manufactured by:Getein Biotechnology Co.,Ltd. based inNanjing, CHINA
Test Item: SARS-CoV-2 Antigen. Sample Type: Human nasal swab. Test Time: 10-15 min. Methodology: Colloidal ...
Manufactured by:Getein Biotechnology Co.,Ltd. based inNanjing, CHINA
Test Item: SARS-CoV-2 Antigen. Sample Type: Human nasal swab. Test Time: 15 min. Methodology: Immunofluorescence ...
Manufactured by:IC Biomedical, LLC based inCartersville, GEORGIA (US) (USA)
Advanced Concept Adsorbent that enables faster charging • Complies with IATA regulations for open cryogenic receptacles Rugged construction and superior vacuum performance with super insulation for maximum holding times. Lockable lid. ...
Manufactured by:MP Biomedicals , LLC based inIrvine, CALIFORNIA (USA)
The Neonatal 17OHP ELISA Kit is intended for the measurement of 17-alpha hydroxyprogesterone (17OHP) in a whole blood ...
Manufactured by:Fluke Biomedical based inCleveland, OHIO (USA)
DPM4 Parameter Tester is a highly accurate meter for testing a wide range of medical devices. Key features include its lightweight, compact size and battery operation. The DPM4 is ideal for the testing done as part of preventive maintenance or repair processes whenever measurement of pressure, flow, or relative humidity is ...
Manufactured by:Fluke Biomedical based inCleveland, OHIO (USA)
The ESA615 Electrical Safety Analyzer brings fast and simple automated testing in the form of a portable analyzer to healthcare technology professionals that perform electrical safety testing on medical equipment both in the field and in facilities. Whether it is simple testing or comprehensive analysis, the ESA615 can do it all. The ESA615 is an all-in-one solution with a safety analyzer and ECG ...
Manufactured by:Ivy Biomedical Systems, Inc based inBranford, CONNECTICUT (USA)
The Model 7800 is Ivy Biomedical System’s 5th generation cardiac trigger monitor used in applications requiring precision ECG R-wave synchronization for gating host systems on and off. The Model 7800 incorporates an intuitive, menu-driven, touch-screen user interface, simplifying operation for clinicians and ...