- Home
- Equipment
- north america
- blood bone
Show results for
Refine by
Blood Bone Equipment Supplied In North America
28 equipment items found
Manufactured by:Alliance Spine based inSan Antonio, TEXAS (USA)
Promote OsteoPro DBM 100 is 100% dehydrated, demineralized bone matrix, processed using a proprietary demineralization process. It is Osteoinductive, Osteoconductive, and Osteogenic when mixed with saline, blood, Bone Marrow Aspirate (BMA), Platelet Rich Plasma (PRP), or other cellular components. ...
Manufactured by:AllCells based inAlameda, CALIFORNIA (USA)
Hematopoietic stem and progenitor cells (HSPCs) are the key stem cell population in the bone marrow capable of self-renewal and multilineage differentiation into mature blood cell types. CD34+ HSPCs can be isolated from mobilized peripheral blood, bone marrow, and umbilical cord blood. Clinical HSPC ...
Manufactured by:Zimmer Biomet CMF and Thoracic based inJacksonville, NEW JERSEY (USA)
The RibFix Advantage Intrathoracic Fixation System is the first and only intrathoracic fixation system for the treatment of rib fractures, which offers a repair that accommodates: Smaller Incisions as compared to a traditional open rib procedure. Minimal disruption to the periosteum helping to preserve the blood supply to the bone. Minimal disruption to the ...
Manufactured by:Humimic Medical based inGreenville, SOUTH CAROLINA (USA)
This Trainer combines unmatched realism with excellent durability to create a hands-on training experience like no other. The model is designed to feel and react like real human tissue, and its self-healing properties allow for repeated use. The model features multiple wound patterns, including a lifelike severe laceration with a bleed that can be stemmed through the application of a tourniquet. ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
Designed to process a mixture of autologous whole blood and bone marrow aspirate, the BioCUE BBMA Concentration System represents an evolution in this technique. The system includes all the components to ASPIRATE blood and bone marrow, easily PROCESS the disposable system, and produce an autologous PRP output* to HYDRATE the ...
Manufactured by:Paragon 28, Inc. based inEnglewood, COLORADO (USA)
The TITAN 3-D™ Wedge System offers a porous titanium wedge that provides an alternative to allograft/autograft bone for an Evans Calcaneal Osteotomy for lateral column lengthening. The TITAN 3-D™ Wedge System builds on Paragon 28®’s portfolio of osteotomy wedges and uses the same patented shapes as the PRESERVE™ Evans Wedges. ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
Remestemcel-L is being developed for inflammatory diseases in children and adults including the treatment of acute graft versus host disease (aGVHD), a potentially life-threatening complication of an allogeneic bone marrow transplant (BMT). Currently, there are no products currently approved in the United States for treatment of steroid-refractory aGVHD in children under 12, and off-label options ...
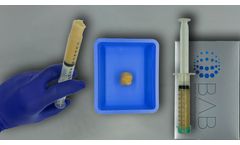
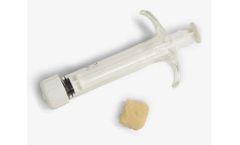
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
H-GENIN Crush-Mix is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Crush-Mix provides a moldable consistency and can be mixed with ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
H-GENIN Putty is intended for use as a demineralized bone matrix for voids or gaps that are not intrinsic to the stability of the bony structure. Putty is indicated for the treatment of surgically created bone defects or bone defects created from traumatic injury. H-GENIN Putty provides a moldable consistency and can be mixed with ...
Manufactured by:Haier Biomedical based inQingdao, CHINA
Applicable for products and samples which require strict storage conditions such as viruses, pathogens, red blood cells, white blood cells, skin, bones, bacteria, semen, biological products, electronics and special materials. Suitable for long term storage applications and compliant with typical storage requirements found in hospitals, disease ...
Manufactured by:phasetwo based inAlpharetta, GEORGIA (US) (USA)
High Capacity High Efficiency Freezers (HCHE) offer advanced solutions for biomedical storage needs, available in capacities ranging from 20,000 to 107,000 vials. These freezers incorporate the latest technology to ensure reliable performance and user-friendly operation. Equipped with automated controls featuring dual-level sensing and touchscreen displays, they provide enhanced product viability ...
Manufactured by:ADS Biotec Inc. based inOmaha, NEBRASKA (USA)
The HANABI-PII Plus reduces hands-on time of the entire harvesting process by 75%, allowing time for other specialized tasks and providing increased throughput for your laboratory. Capable of processing up to 24 samples per run and it eliminates variability compared to manual processing methods. The HANABI-PII Plus can be integrated with additional options such as reagent sensors, waste fluid ...
Manufactured by:Isto Biologics based inHopkinton, MASSACHUSETTS (USA)
Influx™ SPARC is a fully pliable, cellular-enriched allograft with a high concentration of growth factors for use in bone repair or ...
Manufactured by:ADS Biotec Inc. based inOmaha, NEBRASKA (USA)
The HANABI-PI is specifically created for small to mid-size throughput, enabling processing of up to 16 suspension cultures per run and eliminating variability compared to manual processing methods. Walk away reliability and low maintenance reduces FTE time associated with the manual harvesting process up to 75%. The HANABI-PI can be integrated with additional options such as reagent sensors, ...
Manufactured by:EntroGen, Inc. based inWoodland Hills, CALIFORNIA (USA)
Many hematologic malignancies carry characteristic chromosomal translocations believed to play an important role in the pathogenesis of these tumors. Chromosomal translocations found in leukemias and lymphomas either fuse enhancer/promoter regions of one gene to another, causing aberrant expression, or by fusing a part of one gene to that of another, resulting in disruption of the normal function ...
Manufactured by:Tobra Medical Inc. based inWake Forest, NORTH CAROLINA (USA)
The patented Tobra Medical Bone Basket is an innovative surgical device that assists surgeons in maximizing the collection of autologous bone, during bone drilling, for use as a bone graft. The Bone Basket is designed small and compact for in-line collection that is easy to set up. The Bone Basket is provided sterile, and is a single use device that offers an economical value by reducing the ...
Manufactured by:Haier Biomedical based inQingdao, CHINA
A versatile low-temperature freezer installed in hospitals, blood stations, diseases control & prevention centers, research institutions, bioscience laboratories and medical laboratories. It is suitable for storing a wide variety of biological products including viruses, bacteria, red blood cells, white blood cells, skin, ...
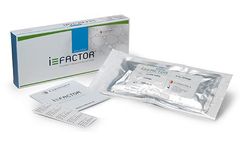
Manufactured by:Cerapedics, Inc. based inWestminster, COLORADO (USA)
i-FACTOR Bone Graft products are intended to replace or augment the use of autologous bone commonly utilized in spine, trauma, and orthopedic procedures such as: Spinal fusion incorporating interbody fusion devices; Treatment of non-union or traumatic fresh fractures; Joint reconstruction requiring a bone void filler. i-FACTOR Bone Graft products are sterilized after packaging (terminally ...
Manufactured by:Crossroads Extremity Systems based inMemphis, TENNESSEE (USA)
The MINIBunion is a minimally-invasive implant and procedure that spares the joint capsule and gives patients a walking recovery instead of being in a cast for several weeks. The minimally-invasive Minibunion® procedure allows bunion repair through a tiny 15mm incision on the side of the foot. The incision spares the joint capsule and is basically not visible after it heals. ...
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
ACTIGEN™ Verified Inductive Bone Matrix is a naturally derived, differentiated allograft matrix with robust handling ...