- Home
- Equipment
- north america
- blood culture
Refine by
Blood Culture Equipment Supplied In North America
24 equipment items found
Manufactured by:Bio Fire Diagnostics based inSalt Lake City, UTAH (USA)
Early identification and treatment of sepsis are essential to combat one of the leading causes of hospital patient deaths.1 With 43 targets, the newly expanded and FDA-cleared BioFire® Blood Culture Identification 2 (BCID2) Panel detects pathogens and antimicrobial resistance genes directly from positive blood ...
Manufactured by:Path-Tec, LLC based inMidland, GEORGIA (US) (USA)
ChloraPrep Frepp 1.5ml Applicator, Latex-Free Adhesive Bandage, (2) Alcohol Prep Pads, Biohazard Bag with Absorbent, 2×2 Sterile Gauze, Latex-Free Tourniquet, Latex-Free Gloves, Polybag ...
Manufactured by:Cepheid based inSunnyvale, CALIFORNIA (USA)
On-demand molecular testing — an ideal solution: Accurate molecular detection of MRSA or SA from a gram-positive blood culture sample in about an hour; Simple test protocol can be run on all shifts to provide results around-the-clock; Provides clinicians actionable information when it is most needed; Easily integrates into your hospital’s bundle of ...
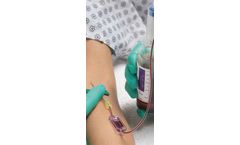
Manufactured by:Kurin, Inc. based inSan Diego, CALIFORNIA (USA)
The patented Kurin Lock with Flash Technology transforms a regular blood culture collection set into a powerful yet simple way to put skin contaminants on the sideline. Each Kurin® collection set features industry-leading butterfly needles and is compatible with all major blood culture bottles. The integrated Kurin Lock® ...
Manufactured by:Accelerate Diagnostics, Inc. based inTucson, ARIZONA (USA)
MALDI prep. Simplified. Automated rapid clean-up of blood cultures for direct ID by ...
Manufactured by:Magnolia Medical Technologies based inSeattle, WASHINGTON (USA)
Steripath Gen2 is the simple, all-in-one solution clinically proven to reduce false positive blood culture results. Reduce your blood culture contamination rate to improve patient safety and key quality outcomes, while significantly reducing unnecessary antibiotic usage, length of stay and hospital ...
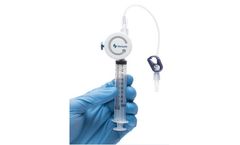
Manufactured by:Magnolia Medical Technologies based inSeattle, WASHINGTON (USA)
With Steripath Micro, you can consistently divert and sequester the skin contaminants known to cause false-positive blood culture results. The latest, low-volume Initial Specimen Diversion Device provides you with a reliable contamination-reducing technology for better patient outcomes and decreased cost burdens from a sepsis ...
Manufactured by:Accelerate Diagnostics, Inc. based inTucson, ARIZONA (USA)
Easier for the lab. Earlier for clinicians. Fast MIC results. Direct from positive blood cultures. Now with optional ID, and affordably ...
Manufactured by:Specific Diagnostics based inSan Jose, CALIFORNIA (USA)
Specific Reveal® Rapid AST system delivers phenotypic AST results in an average of 5 hours, directly from positive blood culture or isolates. Reveal is affordable, high throughput, and provides accurate minimum inhibitory concentration (MIC) results with wide antimicrobial ...
Manufactured by:Dalynn Biologicals based inCalgary, ALBERTA (CANADA)
Our Acridine Orange Stain is used as a fluorescent staining agent to detect the presence of bacteria in blood cultures and other bodily ...
by:T2 Biosystems, Inc. based inLexington, MASSACHUSETTS (USA)
The T2Bacteria® Panel is the first and only FDA-cleared test to identify sepsis-causing bacteria directly from whole blood without the wait for blood ...
Manufactured by:bioMérieux S.A. based inMarcy l`Etoile, FRANCE
60 cell capacity (3,600 blood/body fluids annually), Optimized space saving & cost-effective design – so any laboratory can benefit from BacT/ALERT® 3D technology, Configurable for blood or mycobacterium ...
Manufactured by:Globe Scientific Inc based inMahwah, NEW JERSEY (USA)
Globe Scientific is pleased to offer these premium quality borosilicate glass tubes. The tubes provide excellent chemical resistance and durability. They feature well-rounded bottoms and smooth, fire-polished rims. Dimensional specifications are rigidly controlled for reliable, uniform results. The tubes are produced in an ISO 9001:2008 certified factory. They are available in six sizes. ...
by:T2 Biosystems, Inc. based inLexington, MASSACHUSETTS (USA)
The FDA-cleared and CE-marked T2Dx® Instrument is a fully automated, walk away, clinical multiplex benchtop diagnostic system capable of running tests directly from whole blood. ...
Manufactured by:Anaerobe Systems based inMorgan Hill, CALIFORNIA (USA)
Brain Heart Infusion Broth (BHI BROTH) is an enriched non-selective media intended for the cultivation of most anaerobic bacteria and other fastidious ...
Manufactured by:Infitek Co., Ltd. based inJinan, CHINA
RMX-AR-S is designed to provide stacking rotating plates which doubles the usable surface area; it is precise with speed adjustment and timer functions. It can be placed in an incubator. The mixer is suitable for tissue culture, extraction, blood sample mixing, radioimmunoassay, glucose, in biological, chemical, animal and plant analysis, clinical experiments and ...
Manufactured by:CD Diagnostics based inClaymont, DELAWARE (USA)
Joint infection can occur in a patient with a joint prosthesis (Periprosthetic Joint Infection, or PJI) or a patient without a prosthesis (Native Septic Arthritis, or NSA). Diagnosing joint infection can be challenging and making a comprehensive laboratory workup important to provide a complete view of the potential organism that may be the source of the potential ...
by:T2 Biosystems, Inc. based inLexington, MASSACHUSETTS (USA)
T2MR® technology powers all of the diagnostic innovations at T2 Biosystems. T2MR is a diagnostic detection method utilizing miniaturized magnetic resonance technology which measures how water molecules react in the presence of magnetic fields. Over 200 studies published in peer-reviewed journals have featured T2MR in a breadth of applications, including direct detection and measurement in ...
by:T2 Biosystems, Inc. based inLexington, MASSACHUSETTS (USA)
The T2Cauris™ Panel RUO provides direct detection of the emerging superbug Candida auris in patient skin and patient blood and is now available for research use. The Centers for Disease Control and Prevention (CDC) validated the T2Cauris™ Panel RUO swab test on patient skin samples and published their findings in Mycoses.1 ...
by:Precipio, Inc based inNew Haven, CONNECTICUT (USA)
IV-Cell universal culture media is a Research-use only (RUO), ready-to-use, fully supplemented medium developed specifically to support bone marrow and peripheral blood cell culture for in vitro cytogenetic analysis of hematological disease. IV-Cell is a proprietary culture media that does not require the addition of any ...