- Home
- Equipment
- north america
- blood draw
Refine by
Applications
- Serum Products for Mesenchymal Stem Cells Growth
- Serum Products for Fibroblasts Cell Growth
- Serum Products for Madin-Darby Canine Kidney Cells Growth
- Serum Products for FO Myeloma Cells Growth
- Diagnostic Solutions for Lung Cancer
- Liquid Biopsy Solutions for Breast Cancer
- Diagnostic Solutions for Colorectal Cancer
- Medical Devices for Clinicians
- Medical Devices for Military
- Liquid Biopsy Technology for Hereditary Cancer Screening
- Blood Glucose Meter and Diabetes Supply Products for Diabetes Education
- Blood Glucose Meter and Diabetes Supply Products for Diabetes Education - Exercise and Diabetes
- Blood Glucose Meter and Diabetes Supply Products for Diabetes Education - Exercise Safely
- Blood Glucose Meter and Diabetes Supply Products for Diabetes Education - Meal Planning
Blood Draw Equipment Supplied In North America
200 equipment items found
Manufactured by:Kawasumi Laboratories America, Inc. based inTampa, FLORIDA (USA)
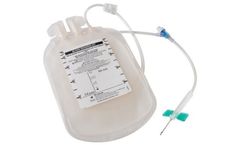
K-Shield Advantage® 600 mL blood bag with 48” tubing, pinch clamp and pre-attached 16G venipuncture needle with safety device, and needle-free sampling ...
Manufactured by:Kawasumi Laboratories America, Inc. based inTampa, FLORIDA (USA)
Provides a safe collection environment for both clinician and patient. Integrated safety device eliminates extra steps for disposal. Fast and simple one-step activation with an “audible” click. Needle is covered during removal, ensuring confidence in safety. Convenience of a pre-attached vacuum tube ...
Manufactured by:Kawasumi Laboratories America, Inc. based inTampa, FLORIDA (USA)
Locking luer connection maintains safety and security, Provides secure specimen sampling, Provides a safe collection environment for both clinician and patient, Self-sealing synthetic needle sheath, Sterile device, Not made with natural rubber ...
Manufactured by:Kawasumi Laboratories America, Inc. based inTampa, FLORIDA (USA)
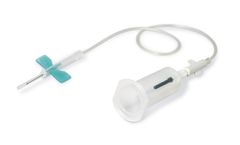
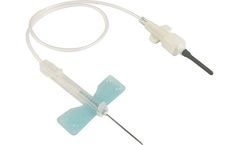
K-Shield Advantage Winged Blood Collection Sets with Multiple Sample Luer Adapter (50 per box / 4 boxes per case). Provides a safe collection environment for both clinician and patient. Integrated safety device eliminates extra steps for disposal. Fast and simple one-step activation with an “audible” click. Needle is covered during removal, ensuring confidence in ...
Manufactured by:Suneva Medical based inSan Diego, CALIFORNIA (USA)
Concentration system uses a unique separating gel to greatly improve the collection of PRP. Yields the most platelets per mL from the smallest blood draw. Designed to accommodate busy practices. Amplifine PRP is the quickest and most efficient concentrating system available. ...
Manufactured by:Spectros Medical Devices Inc. based inHouston, TEXAS (USA)
The oral sensor is designed to fit in the mouth of adults to neonates to monitor the saturation of the buccal mucosa, as a surrogate for GI perfusion. This information prevents excessive blood draws and provides valuable information to manage cardiac output and the arteriovenous oxygen ...
Manufactured by:Neo Medical, Inc. based inSparks, NEVADA (USA)
Neo Medical’s ‘break and drop’ introducer needle still plays an important role in difficult placements, and the large, clear window provides rapid confirmation of blood draws. Non-Safety. ...
Manufactured by:Tempo Medical Products based inPhoenix, ARIZONA (USA)
Lightly textured to provide maximum control; Innovative design prevents tourniquet from slipping or unraveling; Recommended for single patient use, to help reduce the risk of cross-contamination; Used by phlebotomists to initiate blood draws and begin the apheresis process; Ideal product for blood donor and IV starter ...
Manufactured by:Delta Med based inViadana, ITALY
The Delta Med P.Valve is among the leading products in the Needleless Connectors category. Totally transparent to assist blood draws, it supports up to 600 activations guaranteed for 7 days. The valve is light and flat, with a swabable surface, and is suitable for neonatal infusions and oncology (flow rates 160ml per ...
Manufactured by:Tempo Medical Products based inPhoenix, ARIZONA (USA)
The non latex material will safeguard patients by eliminating latex related allergic reactions; Innovative design prevents tourniquet from slipping or unraveling; Used by phlebotomists to initiate blood draws and begin the apheresis process; Ideal product for blood donor and IV starter kits; Conveniently packed flat for ease of use and simple ...
by:Natera, Inc. based inAustin, TEXAS (USA)
These conditions, which affect quality of life, could benefit from early intervention and might otherwise go undetected. Vistara tests for 25 serious genetic conditions with a blood draw from the mother. Most results will be returned to a clinician within 2-3 ...
Manufactured by:Garware Fulflex USA Inc. based inBrattleboro, VERMONT (USA)
Our tourniquets are free of latex protein allergens, non-slip, tear resistant and meet current toxicology requirements. They apply even pressure around the limb, making them perfect for phlebotomy and safe for blood drawing ...
Manufactured by:SRI Healthcare based inAtlanta, GEORGIA (US) (USA)
Isolation Gowns are a Non-Surgical medical device intended to protect the wearer from the transfer of microorganisms and body fluids in low or minimal risk patient isolation situations such as blood draw, suturing, in the Intensive Care Unit (ICU), or a pathology lab. Let SRI help your hospital staff stay safe by setting up a convenient Processing & Tracking ...
Manufactured by:Merit Medical Systems based inSouth Jordan, UTAH (USA)
The Prelude ACT (Activated Clotting Time) sheaths are part of Merit’s comprehensive family of vascular access products. These half-size sheaths allow easy blood draw through sheath side arm with or without catheters in place. The ACT sheaths accommodate a variety of catheters and other interventional devices to meet your clinical needs. ...
Manufactured by:IGeneX based inMilpitas, CALIFORNIA (USA)
For most patients, ordering a Blood Collection Kit is the first step in getting tested by IGeneX for Lyme disease, Tick-Borne Relapsing Fever, or other tick-borne diseases. We also offer a Urine Collection Kit and Miscellaneous Collection Kit, but most tick-borne disease tests offered by IGeneX are compatible with the Blood Collection Kit. Please note that the ...
Manufactured by:THE SUTURE BUDDY based inSpring, TEXAS (USA)
Our most comprehensive kit so far & A free how to video on Lidocaine Infiltration! - Video available for download after checkout. Our latest kit allows you to practice your Venipuncture and Blood Draw skills as well as your suture skills! Kit Includes: Venipuncture Pad: Pad Dimensions: 7" x 4.5" x 0.5", 3mL Syringe with 23G Needle, Box of 3-0 Sutures (12ct), ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Covered by Medicare, Prospera is a transplant rejection assessment test that uses a simple blood draw to evaluate the risk of rejection of a transplanted kidney. Through the use of advanced cell-free DNA technology, Prospera increases a provider’s ability to identify otherwise undetected rejection that might lead to kidney loss. ...
Manufactured by:Guardant Health, Inc. based inRedwood City, CALIFORNIA (USA)
Our Guardant360® CDx test is FDA-approved for complete genomic testing across all solid cancers, providing doctors guideline-complete genomic results in 7 days from a simple blood draw to inform treatment decisions. A blood test does not require tissue testing, enabling more patients to benefit from the growing number of FDA-approved targeted ...
Manufactured by:AIVITA Biomedical, Inc. based in, CALIFORNIA (USA)
AIVITA Biomedical is using its patient-specific vaccine platform to develop a rapidly scalable vaccine candidate, AV-COVID-19, for the novel coronavirus (SARS-CoV-2). AIVITA’s personalized vaccine begins with a basic blood draw, from which the recipient’s own immune cells are extracted and loaded with SARS-CoV-2 antigen. The resulting personalized ...
Manufactured by:Hemostemix Inc. based inCalgary, ALBERTA (CANADA)
Hemostemix’s technology uses a patient’s own cells to treat that patient’s disease. The cornerstone of this autologous technology is a novel cell population within the blood called the synergetic cell population (SCP). The synergetic cell population, which can be collected from a simple blood draw, consists of progenitor and ...