- Home
- Equipment
- north america
- blood flow restoration
Show results for
Refine by
Blood Flow Restoration Equipment Supplied In North America
9 equipment items found
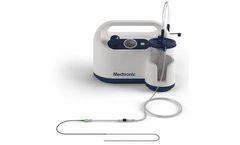
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Riptide Aspiration System is designed to retrieve a blood clot and restore blood flow to occluded vessels in the brain for patients experiencing acute ischemic stroke (AIS) due to a large vessel occlusion ...
Manufactured by:Cerevast Medical, Inc. based inBothell, WASHINGTON (USA)
Pre-clinical and clinical studies of microsphere-mediated ophthalmic ultrasound have shown encouraging results as a non-invasive treatment for retinal vein occlusion leading to restored blood flow, reduction in intra-retinal hemorrhage volume and improved visual acuity ...
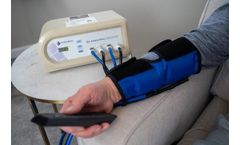
by:BioTAB Healthcare based inSaint Louis, MISSOURI (USA)
The Bio Arterial Plus is a non-invasive, in-home treatment clinically proven to reduce the risk of amputation and symptoms of PAD. The Bio Arterial Plus is a non-invasive arterial enhancement system that’s clinically proven to increase arterial blood flow, promote wound healing, and reduce pain and discomfort associated with Peripheral Arterial Disease ...
Manufactured by:Orchestra BioMed, Inc. based inNew Hope, PENNSYLVANIA (USA)
Virtue SAB is a patented drug/device combination product for the treatment of artery disease that delivers a proprietary extended release formulation of sirolimus, SirolimusEFR, during balloon angioplasty without the need for a coating or a permanent ...
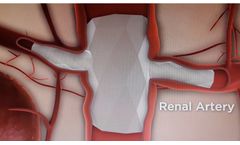
Manufactured by:Red Vascular Technology, LLC based inTampa, FLORIDA (USA)
Red Vascular’s non-modular, branched endoprosthesis represents an ideal solution for aortic aneurysmal disease with branch artery involvement. Its design and delivery through a single femoral artery site are superior to modular, fenestrated grafts currently used to treat complex aneurysms. Fenestration adds several complex steps to deployment of multiple grafts and leads to ...
Manufactured by:Cerevast Medical, Inc. based inBothell, WASHINGTON (USA)
When a blood clot or thrombus is formed, strands of fibrin anchor one red blood cell to another. It is these fibrin strands that form the lattice or structural matrix of the blood clot. The formation or deposition of these clots in the arterial vessels of the brain is one of the primary causes of ischemic stroke. Intravenous thrombolytic therapy (tPA/alteplase, the only approved drug for the ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Solitaire X revascularization device, featuring Parametric™ design, a unique overlapping stent retriever-based technology, restores blood flow and retrieves clots from occluded blood vessels in the brain for patients experiencing acute ischemic stroke (AIS) due to a large vessel occlusion ...
Manufactured by:RepliCel Life Sciences based inVancouver, BRITISH COLUMBIA (CANADA)
RCT-01 is a proprietary autologous cell therapy being developed to treat chronically damaged tendons. * *This product is currently in clinical testing and not yet commercially available. RCT-01 is an autologous cell therapy utilizing non-bulbar dermal sheath (NBDS) cells - a type of fibroblast cell - isolated from the hair follicle to repair and regenerate tissue. Though several cell types are ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Imatinib Mesylate (STI571 Mesylate) is a tyrosine kinases inhibitor that inhibits c-Kit, Bcr-Abl, and PDGFR (IC50=100 nM) tyrosine ...