- Home
- Equipment
- north america
- bone development
Show results for
Refine by
Bone Development Equipment Supplied In North America
15 equipment items found
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
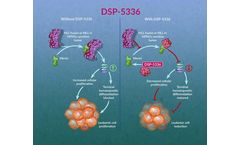
Menin is a scaffold nuclear protein that plays various key roles in biological pathways, including cell growth regulation, cell cycle control, genomic stability, bone development, and hematopoiesis.26,27 Binding of menin to MLL fusion and wildtype proteins leads to the upregulation of HOXA family and MEIS1 genes that function to stall myeloid cellular ...
Manufactured by:EpiBone, Inc. based inBrooklyn, NEW YORK (USA)
It means building a diverse team of expert scientists and entrepreneurs—highly credentialed people committed to always keep learning and never compromise on quality—then harnessing the creative power of those diverse minds. That’s why our mission rests on growing thriving people as well as bone, developing best practices in business to ...
Manufactured by:MicroAire Surgical Instruments, LLC. based inCharlottesville, VIRGINIA (USA)
The ergonomic Solo™ Driver is the smallest wire driver with the widest range of wire sizes, part of the new 5000 Series small-bone power system developed with surgeons, for surgeons by MicroAire. 5641 Modular Trauma Instrument System. 5000ET Small-Bone ...
Manufactured by:Centinel Spine based inWest Chester, PENNSYLVANIA (USA)
Clinical Applications: Addressing Crucial Clinical Needs. STALIF L™ continues the 25+ year STALIF® heritage of innovation. Developed with prominent spine surgeons to address an unmet need, STALIF L meets the demands and complexity of minimally invasive lumbar lateral interbody fusion procedures. Surgical Ease: Integrated self-tapping, self-drilling screws simplify implant immobilization ...
Manufactured by:Rocket Pharmaceuticals, Inc. based inCranbury, NEW JERSEY (USA)
Approximately two-thirds of FA cases are caused by genetic defects in the FANCA gene, which results in the FA subtype known as FA Complementation Group A (FA-A). FA patients may develop bone marrow failure (very low blood counts), cancers of the blood or other ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
OsteGro V Osteo Viable Matrix is the Viable Bone Matrix (VBM) product in the OsteGro family. It is comprised of cancellous bone particles with preserved cells combined with demineralized cortical particles and fibers all processed via Aziyo’s proprietary gentle VBM processes. OsteGro Allograft Bone Matrix, the second product in this family, is a sterile bone matrix comprised of cancellous ...
Manufactured by:Cell Applications, Inc. based inSan Diego, CALIFORNIA (USA)
Instead, chondrocytes undergo terminal differentiation and eventually apoptose, leading to mineralization of cartilage in a process resembling bone formation during development. Thus, HC-OA provide a useful model to study changes in chondrocyte biology in response to abnormal environment of the OA joint. HC-OA from Cell Applications, Inc. have been used to ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
ViBone is comprised of cancellous bone particles with preserved cells and demineralized cortical bone particles produced via Aziyo’s gentle proprietary VBM ...
Manufactured by:Isto Biologics based inHopkinton, MASSACHUSETTS (USA)
Influx™ SPARC is a fully pliable, cellular-enriched allograft with a high concentration of growth factors for use in bone repair or ...
by:Oxitone Medical based inHartford, CONNECTICUT (USA)
Oxitone 1000M - World’s First FDA-cleared Wrist-Sensor Pulse Oximetry Monitor; No fingertip probe, Bluetooth, Rechargeable long life battery., Wearable, easy to use, FDA-cleared, CE Mark ...
Manufactured by:Aziyo Biologics, Inc. based inSilver Spring, MARYLAND (USA)
Viable Bone Matrix for Spine and Orthopedic Procedures. Fiber Viable Bone Matrix: Fiber Viable Bone Matrix (VBM) is made of cancellous bone particles with preserved cells combined with demineralized cortical fiber produced via Aziyo’s gentle proprietary VBM ...
Manufactured by:Biogennix based inIrvine, CALIFORNIA (USA)
Agilon Strip Collagen Enhanced Bone Grafting Product is the latest addition to the Biogennix bone grafting product family. Developed with Biogennix proprietary TrelCor technology, Agilon Strip consists of resorbable, osteoconductive TrelCor granules densely packed in a matrix of type 1 bovine ...
Manufactured by:Everlywell, Inc. based inAustin, TEXAS (USA)
Our Vitamin D3 Supplement powers immunity and supports strong bones and muscle health. Enhance your wellness routine with our gluten-free, vegetarian ...
Manufactured by:Standard Process Inc. based inPalmyra, WISCONSIN (USA)
Calcium Lactate is a dairy-free, vegan tablet that helps maintain healthy bone density.* It is an excellent source of calcium and a good source of ...
Manufactured by:Himed based inOld Bethpage, NEW YORK (USA)
Beta-tricalcium phosphate (β-TCP) is a calcium phosphate biomaterial noted for its bioactive and resorbable properties, which make it highly effective in applications such as bone grafting and implants. It supports bone regeneration due to its osteoconductive nature, acting as a scaffold for natural bone to replace over time. By adjusting the porosity and particle size of β-TCP, its ...