- Home
- Equipment
- north america
- cancer immunotherapeutics
Refine by
Cancer Immunotherapeutics Equipment Supplied In North America
45 equipment items found
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
The gene-edited iPSC will then be used to produce iMSC that have been endowed with beneficial properties to broaden and enhance therapeutic uses. Our first gene-edited iMSC product will be used as a cancer immunotherapeutic, taking advantage of the tumor homing properties of MSC and engineering the cells to deliver immune-stimulating proteins to enhance the ...
by:Metaclipse Therapeutics Corporation based inAtlanta, GEORGIA (US) (USA)
Influenza (Target age 65+) and Influenza+ SARS-C0V-2 ...
by:Calviri based inPhoenix, ARIZONA (USA)
Calviri’s immunotherapy diagnostic is a huge leap forward in predicting therapy outcomes compared to current available ...
by:Verb Biotics based inBoston, MASSACHUSETTS (USA)
Yso5 is a discovery-stage program leveraging a specific keystone gut bacteria (undisclosed) to potentiate the effects of Immune Checkpoint Blockade (ICB) drugs. The field of cancer therapy has been revolutionized by the use of immunotherapy. The microbiome breakthrough science today paves the way for innovative biotherapies to potentiate the effectiveness of these cancer immunotherapies. YSOPIA ...
by:Enveric Biosciences, Inc. based inNaples, FLORIDA (USA)
Enveric Biosciences seeks to advance novel combinations of CBD with chemotherapeutic agents and immunotherapies. Preclinical data suggests combination therapies may improve the activity of certain chemotherapies or cancer immunotherapies, potentially enabling more potent or longer-lasting therapeutic effects. In addition, combination therapies may allow for the same or greater therapeutic effect ...
Manufactured by:JPT Peptide Technologies GmbH based inBerlin, GERMANY
Neo-epitopes are important targets for individualized cancer immunotherapy. Recent advancements in next generation sequencing and bioinformatic approaches to predict immunogenicity of neo-epitopes improved target selection for therapy. However, in many cases only a fraction of predicted epitopes generate a specific T-cell response. Therefore, various assay techniques such as ELISpot, ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
B7H3 belongs to the B7 family of immune checkpoint inhibitors and is expressed on a wide range of solid and hematologic malignancies. B7H3 is an important mediator of tumor angiogenesis and metastasis, and higher expression is associated with a poor prognosis for patients. We are developing FT573, an investigational, universal, off-the-shelf NK cell cancer immunotherapy derived from a clonal ...
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
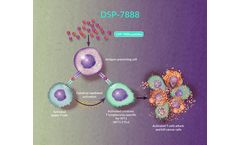
Ombipepimut-S Emulsion (DSP-7888) is an investigational immunotherapeutic cancer vaccine containing 2 peptides that induce WT1-specific cytotoxic T lymphocytes (WT1-CTLs) and helper T cells to attack WT1-expressing cancerous cells found in various types of hematologic malignancies and solid tumors.1,2 Researchers have identified that adding ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
In addition to CD38 targeting in multiple myeloma, targeting of other tumor-associated cell-surface proteins has been clinically investigated. Of these antigens, the TNF-superfamily member B-cell Maturation Antigen (BCMA) is among the most researched. Several clinical trials in multiple myeloma have shown promising initial results targeting BCMA with CAR T cells. ...
Manufactured by:Immune-Onc Therapeutics, Inc. based inPalo Alto, CALIFORNIA (USA)
Immune-Onc is evaluating IO-108, an antagonist antibody targeting the myeloid checkpoint, LILRB2 (also known as ILT4). The U.S. FDA has cleared the IND application for IO-108 as a potential therapy for solid tumors. The company expects to begin clinical trials with IO-108 in the second half of ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
MICA and MICB (MICA/B) are stress proteins expressed on a broad range of solid and hematopoietic tumor cells and serve as an activation signal for NKG2D+ NK and T cells. However, MICA/B are frequently shed as an immune escape mechanism, preventing recognition and destruction of tumor cells by the immune system. Proteolytic shedding of MICA/B can be inhibited by monoclonal antibodies targeting the ...
Manufactured by:MedGenome Inc. based inCounty of New Castle, DELAWARE (USA)
OncoPeptTUME — A novel in-silico approach to model the tumor microenvironment and predict treatment efficacy and long-term survival benefits for immunotherapy applications. Cancer immunotherapy is now established as a major therapeutic modality. Cancer immunotherapy drugs elicit their anti-tumor immune response in a subset of the treated patients by activating CD8 T-cells and provide ...
Manufactured by:Tactiva Therapeutics based inBuffalo, NEW YORK (USA)
Adoptive Cell Transfer (ACT) is a form of immunotherapy that targets tumors specifically and potently by utilizing large numbers of a patient’s own immune cells to target and destroy cancer cells. Typically, starting with a few immune cells derived from the patient’s blood, billions of cancer fighting immune cells are manufactured in a special facility, and given back to the ...
Manufactured by:Greenwich LifeSciences, Inc. based inHouston, TEXAS (USA)
Following breast cancer surgery, a HER2/neu 3+ patient receives trastuzumab (Genentech/Roche’s Herceptin, a monoclonal antibody treatment that targets the HER2/neu protein) in the first year and then hopes that their breast cancer will not recur, with the odds of recurrence slowly decreasing over the first 5 years. Our immunotherapy, GP2, may be synergistic with Herceptin. GP2 is a nine ...
Manufactured by:Caprico Biotechnologies, Inc. (CBI) based inNorcross, GEORGIA (US) (USA)
The clone 528, a mouse monoclonal antibody, specifically reacts with an epitope of the ~170 kDa extracellular protein domain of human epidermal growth factor receptor or commonly known as EGFR. Physiologically EGFR is expressed in the skin, gastrointestinal system, kidney, and other normal tissues as well as aberrantly over expresses in epithelial cancer cells of lung, pancreas, colon, breast, ...
Manufactured by:Elpiscience based inShanghai, CHINA
ES002’s dual ATP-adenosine mechanism is a potentially powerful TME regulator and transformative cancer immunotherapy. In preclinical studies ES002 demonstrated significant single agent anti-tumor activity as well as an excellent tolerability profile. ES002 has also shown superior enzymatic inhibition and binding ...
by:Oncimmune Holdings PLC based inNottingham, UNITED KINGDOM
Oncimmune’s proprietary biomarker discovery engine, which is leveraged in our ImmunoINSIGHTS services to discover and validate novel biomarkers that can help stratify patients in multiple cancer indications and with different autoimmune ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Why human derived? Brooklyn has developed a powerful method to create complex cytokine mixtures derived from a human source. Cytokines are powerful immune factors that play an essential role in T cell signaling, activation, proliferation, and tumor-fighting responses. We know cytokines may induce beneficial reactions in cancer patients. To date, available cytokine therapies have been recombinant ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
Multiple myeloma is a hematologic malignancy characterized by the proliferation of malignant plasma cells. In multiple myeloma, malignant plasma cells accumulate in the bone marrow and produce abnormal antibodies called M proteins, which can cause kidney damage, bone destruction, and impaired immune function. While multiple approved drugs with novel mechanisms have improved disease management ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
CAR T-cell therapy has recently emerged as a revolutionary and potentially curative therapy for patients with certain hematologic malignancies, including refractory cancers. In 2017, two CAR T-cell therapies were approved by the FDA for the treatment of relapsed / refractory B-cell precursor acute lymphoblastic leukemia (ALL) and relapsed / refractory diffuse large B-cell lymphoma (DLBCL). While ...