- Home
- Equipment
- north america
- catheter manufacturing system
Show results for
Refine by
Catheter Manufacturing System Equipment Supplied In North America
49 equipment items found
Manufactured by:Vante, Brand of Machine Solutions Inc. based inTucson, ARIZONA (USA)
The Vante RUBY System is an improved catheter manufacturing system delivering high reliability, throughput and yields along with an improved control interface for superior automation integration. Enhanced functionality for RUBY include “Smart Mold”, a patented feature, provides the ability for parametric data to be ...
Manufactured by:Vante, Brand of Machine Solutions Inc. based inTucson, ARIZONA (USA)
The Vante Onyx catheter manufacturing system is designed to maximize production output while minimizing operating expense in high volume production environments. This system incorporates production capabilities of up to four (4) independently controlled Vante tipping stations into a compact table top platform which can be ...
Manufactured by:Vante, Brand of Machine Solutions Inc. based inTucson, ARIZONA (USA)
The Vante Jade Catheter Manufacturing System is designed as a flexible catheter manufacturing platform targeting the mid-range market where the customer designs the molds and develops the application, production cycle times and throughput are important, and where precision thermal profiles and flash free output ...
Manufactured by:MASS Medical Storage based inLenexa, KANSAS (USA)
FIFO Glide™ Catheter Storage – Exceeds industry average storage ...
Manufactured by:Vante, Brand of Machine Solutions Inc. based inTucson, ARIZONA (USA)
Today’s catheter delivery systems rely on increasingly complex shapes, constructions and polymers to deliver their intended treatment. Increasing complexity in catheter bonding and welding applications can only be consistently achieved through advanced technology and proper application of sound engineering principles. Vante and PlasticWeld Systems equipment is the global standards for ...
Manufactured by:SRS Medical based inN. Billerica, MASSACHUSETTS (USA)
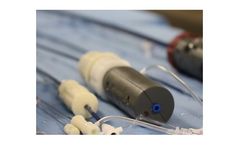
Knowing when to remove a Foley has always been a challenge. Doing it without disturbing the catheter was impossible… until now. Attaching directly to an existing Foley catheter, the device works by using the catheter balloon to measure changes in vesical pressure during both the filling and emptying ...
Manufactured by:TruCath Catheters. A division of HR Pharmaceuticals, Inc. based inYork, PENNSYLVANIA (USA)
The TruCath Closed System Catheter contains a pre-lubricated catheter and attached collection bag for a no-touch catheterization procedure. The introducer tip shields the catheter from bacteria in the beginning of the urethra where most bacteria are concentrated. Sold as a sterile, single-use catheter/bag ...
Manufactured by:MASS Medical Storage based inLenexa, KANSAS (USA)
Lucent™ High Density Modular Storage – Optimize your work space within our exchange storage system. With our transparent Lucent™ High Density Modular Storage supply trays, you can customize each supply tray quickly and easily. Keep your supplies organized, streamlined, and easily ...
Manufactured by:Symple Surgical Inc. based inFlagstaff, ARIZONA (USA)
The Symple Surgical GRIZZLYTM microwave ablation catheter system is a beside-the-scope, over-the-wire, moveable antennae, compliant urethane balloon system. The catheter is a fully automated system that can create complete circumferential and spot ablations from a single device up to 40mm in length. A compliant urethane balloon provides the ability to target esophagus diameters between 18-35mm. ...
Manufactured by:Resolution Medical based inFridley, MINNESOTA (USA)
Finished Devices & Components: Multi-Catheter Systems. Valve Delivery & Repair. Stent Delivery Systems. Steerable Technologies. Tipping and Over-molding. Handle Design & Assembly. Rail/Guide Systems. Vertical and Horizontal ...
Manufactured by:S2S Global based inCharlotte, NORTH CAROLINA (USA)
Our NanoCath TrueSafe Comfort® Line in the PremierPro™ by Medsource Family of Catheters provides you the latest technology for your tiniest patient. We offer 24G and 26G with two lengths so that you can have better chance of success your first try. Our push button activated safety features also help reduce the risk of accidental needlesticks injuries. Each catheter is equipped with our ...
Manufactured by:Stereotaxis, Inc. based inSt. Louis, MISSOURI (USA)
The Vdrive Robotic Navigation System with Niobe ES magnetic navigation system provides breakthrough navigation and stability for diagnostic and ablation devices designed with key features to assist in the delivery of better ablations. ...
Manufactured by:MASS Medical Storage based inLenexa, KANSAS (USA)
Hanging Cath Storage Cabinets – Storage space unrestricted by horizontal limits. 22” D x 40-7/8” W x 89-1/4” H. Utilize the FIFOGlide™ Catheter Management System to make the most of your cath lab storage space. Catheters hang vertically on our slide-out FIFOGlide™ tracks, inventory management smooth and ...
Manufactured by:Infraredx, Inc. based inBedford, MASSACHUSETTS (USA)
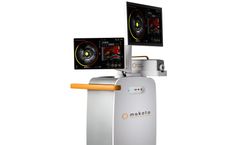
There are still broad swaths of uncharted territories concerning the role lipid core plaques play in heart disease. Now, you can achieve unparalleled insights with the Dualpro™ IVUS+NIRS catheter and its accompanying Makoto Intravascular Imaging System, the only FDA-cleared dual-modality catheter and imaging system indicated for the detection of lipid core plaque (LCP) and the ...
Manufactured by:S2S Global based inCharlotte, NORTH CAROLINA (USA)
Our ClearSafe Comfort® Line in the PremierPro™ by Medsource Family of Catheters provides you the latest technology without having to sacrifice your preferred safety style. Select from a range of gauges and lengths that offer you the best in Safety and Blood Control. Our slide-activated safety features also help reduce the risk of accidental needlestick injuries. Each catheter is ...
Manufactured by:Pepex Biomedical, Inc. based inNorcross, GEORGIA (US) (USA)
According to the Centers for Disease Control and Prevention (CDC), Sepsis is the tenth most common cause of death overall in the U.S. Sadly, there is no commercially available test that actually detects the sepsis bacteria and current clinical protocols are simply a process of ...
Manufactured by:Nordson Medical based inHuntington Beach, CALIFORNIA (USA)
Starting with your clinical requirements, our engineers can work with you to design and manufacture high-performance medical reinforced tubing with the performance characteristics you ...
Manufactured by:Nextern Inc based inWhite Bear Lake, MINNESOTA (USA)
We specialize in the design, development, and manufacturing of complex, highly specialized catheters, steerable devices, and delivery systems for interventional markets. We’ve worked with partners on steerable balloon catheters, steerable neurovascular devices, and delivery devices for structural heart implants. ...
Manufactured by:Penumbra, Inc. based inAlameda, CALIFORNIA (USA)
The Penumbra JET™ D Reperfusion Catheter is intended for use in the revascularization of patients with acute ischemic stroke secondary to large vessel occlusion. The Penumbra JET D is designed to navigate smaller, distal vessels and extract clot efficiently with the power of the Penumbra ENGINE™ aspiration ...
Manufactured by:PFM Medical, Inc. based inCarlsbad, CALIFORNIA (USA)
The Nit-Occlud PDA occlusion system is the ideal choice for small to medium sized patent ductus arteriosus types. The tightly compact nitinol windings provide safe and effective closure for its patients. This highly flexible and adaptive coil system comes pre-loaded in the delivery system is easily repositionable and retrievable to benefit ...