- Home
- Equipment
- north america
- chronic acute wounds
Show results for
Refine by
Chronic Acute Wounds Equipment Supplied In North America
51 equipment items found
Manufactured by:Integra LifeSciences based inPrinceton, NEW JERSEY (USA)
MediHoney, with Active Leptospermum Manuka Honey (ALH), is the global leading medical-grade honey-based product line for the management of wounds and burns. As the most studied variety of medical-grade honey, with over 200 pieces of clinical evidence, these products have become a first-line choice among many clinicians to help in the management of acute, ...
Manufactured by:Integra LifeSciences based inPrinceton, NEW JERSEY (USA)
MediHoney, with Active Leptospermum Honey, (ALH) is the global leading medical-grade honey-based product line for the management of wounds and burns. Derived from the Leptospermum species of tea tree, these unique dressings have properties that can be beneficial throughout all phases of the wound healing. Due to its properties, MediHoney products have become a ...
Manufactured by:Wright Medical Group N.V. based inMemphis, TENNESSEE (USA)
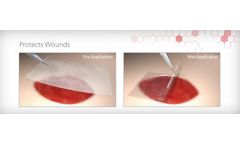
Preserves native amniotic tissue proteins and protects acute and chronic wounds*, Tissue Thickness: ~200μm, Easy to see and manipulate, Omni-directional application, Wet or dry application, Room temperature ...
Manufactured by:Advanced Oxygen Therapy Inc. based inOceanside, CALIFORNIA (USA)
Are you doing everything you can for your patients? Chronic wounds can debilitate your patients for years, causing interruptions to not only their health but their lives and everything they enjoy. Now you can help get your patients back on track and put an end to their suffering. AOTI’s unique Topical Wound Oxygen (TWO2) therapy is a safe, non-invasive, adjunctive multimodality that helps ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Matrix HD Allograft Dermis is an acellular human dermis allograft sterilized to a Sterility Assurance Level (SAL) of 10-6 using the Tutoplast Tissue Sterilization Process. This proprietary process retains the three-dimensional intertwined multidirectional fibers and mechanical properties of the native dermis tissue. The Matrix HD graft provides a natural scaffold to support the body's ...
Manufactured by:Acera Surgical Inc. based inLouis, MISSOURI (USA)
For clinicians with patients requiring soft-tissue repair, Restrata is a non-biologic, hybrid-scale fiber matrix engineered to be similar to human ECM that activates granulation tissue in refractory wounds while maintaining tensile strength with ...
Manufactured by:Unilene based inFort Myers, FLORIDA (USA)
Primary dressing of wounds from moderate to severe, including chronic wounds, leg ulcers, wounds with a little bleeding, areas with fungal lesions, abrasions, lacerations and post surgical ...
Manufactured by:ConvaTec Inc. based inBerkshire, UNITED KINGDOM
Light and flexible every day: The FoamLite™ dressing is specifically designed for the management of low to non-exuding chronic and acute wounds. The dressing is a thin foam dressing with a breathable film and perforated, gentle skin friendly silicone adhesive.†1 FoamLite™ ConvaTec dressing is flexible and conforms†1 to ...
Manufactured by:Canadian Hospital Specialties Limited (CHS) based inOakville, ONTARIO (CANADA)
ActiGraft System, a patented proprietary technology was developed by RedDress, a privately held company based in Israel. Their goal is to develop more effective, natural and economically viable treatments for Chronic Wounds. The first of its kind technology enables health care providers to produce in real time – an in vitro blood clot from a patient’s whole blood. Once applied the ...
Manufactured by:Sano Diagnostics LLC based inWellesley Heights, MASSACHUSETTS (USA)
Simplifying detection of Inflammatory Markers relevant to multiple diseases. SANO Diagnostics’ technology is founded on the ability to detect certain markers of inflammation, a class of proteins known as ...
Manufactured by:Milliken & Company based inSpartanburg, SOUTH CAROLINA (USA)
AGILE dressings were developed to meet the unmet needs of patients and clinicians for a highly absorbent gellable dressing with increased structural integrity and conformability. AGILE dressings combine the patented Active Fluid Management® technology with an innovative weave of gelling fibrils. AGILE has the unique ability to pull excess moisture away from the wound bed, absorbs it into the ...
Manufactured by:AVITA Medical based inValencia, CALIFORNIA (USA)
Epidermolysis Bullosa (EB) is a group of rare and incurable skin disorders caused by mutations in genes encoding structural proteins resulting in skin fragility and blistering, leading to chronic wounds and, in some sub-types, an increased risk of squamous cell carcinoma or death. Dystrophic EB is estimated to affect 3-8 million people[1], and there is currently no FDA-approved ...
Manufactured by:Avery Dennison Medical based inChicago,, ILLINOIS (USA)
Superabsorbent dressing is a sterile, superabsorbent wound dressing for use on wounds with high to excessive amounts of exudate. The dressing consists of a white, superabsorbent core, a clear, perforated, polyethylene film wound contact layer and a white, hydrophobic nonwoven backing, which together help minimize moisture leakage. The dressing absorbs exudate while helping maintain a moist wound ...
Manufactured by:Gel4Med, Inc. based inAllston, MASSACHUSETTS (USA)
Our first product is a flowable extracellular matrix that promotes infection free tissue regeneration in surgical, chronic, and combat wounds without the use of any exogenous added drugs or ...
Manufactured by:Smith & Nephew plc based in, UNITED KINGDOM
Whether you’re a surgeon who’s just been presented with a new case, a nurse treating a chronic wound or working in acute care, it’s your responsibility to stay in control of infection ...
Manufactured by:SweetBio based inMemphis, TENNESSEE (USA)
APIS®, the first product made with MX Technology, is a bioengineered wound product for the management of chronic and acute wounds. APIS covers and protects the wound, absorbs exudate, and creates a moist wound environment. Invitro data demonstrates that APIS reduces bacterial load, decreases ...
Manufactured by:Misonix based inFarmingdale, NEW YORK (USA)
The Next Generation of Amniotic Tissue for Chronic Wound Care. Therion is a dehydrated and terminally sterilized allograft wound covering derived from human placental membrane. Unlike other amniotic membranes on the market, the proprietary ChAmPion process removes the maternal-derived decidua cells, leaving the amnion and chorion layers in their native configuration. By maintaining the native ...
Manufactured by:Medline Industries, LP based inNorthfield, ILLINOIS (USA)
Nonwoven dressing with Hydrolock technology and antimicrobial action designed to gel in contact with exudate for sustained protection against broad range of gram-negative and gram-positive bacteria. Hydrolock technology helps lock in fluid to reduce risk of leakage and maceration. High-retention capacity helps absorb and retain exudate, bacteria and blood to reduce risk of leakage onto ...
Manufactured by:Sanara MedTech Inc. based inFort Worth, TEXAS (USA)
HYCOL HYDROLYZED COLLAGEN provides the benefit of hydrolyzed collagen fragments that are approximately 1/100th the size of native collagen. Give your patients a head start toward wound resolution with HYCOL® HYDROLYZED COLLAGEN. With HYCOL® HYDROLYZED COLLAGEN, the body does not have to break it down, so the benefits of collagen are provided to the ...
Manufactured by:GRAFIX PL, by Smith+Nephew, Inc. based inColumbia, MARYLAND (USA)
GRAFIX PLUS Placental Membrane is designed for advanced wound management, focusing on treating chronic wounds. This product leverages human placental membranes, which are minimally manipulated to retain their native cells, growth factors, and extracellular matrix, ensuring the preservation of structural integrity. The membrane comprises three essential components of the placental structure, ...