- Home
- Equipment
- north america
- clinical regulations
Refine by
Applications
- Automatic tablet & capsule counter solutions for pharmacy industry
- Automatic tablet & capsule counter solutions for pharmaceutical industry
- Automatic tablet & capsule counter solutions for medical supply industry
- Automatic tablet & capsule counter solutions for medical and weight loss Clinics industry
- Automatic tablet & capsule counter solutions for education industry
- Automatic tablet & capsule counter solutions for government & law enforcement industry
- Automatic tablet & capsule counter solutions for military industry
- Automatic tablet & capsule counter solutions for rehab facilities industry
Clinical Regulations Equipment Supplied In North America
30 equipment items found
Manufactured by:Bisco, Inc. based inSchaumburg, ILLINOIS (USA)
Manufactured by:Trio Ostomy Care, Trademark of Trio Healthcare Ltd based inPrinceton, NEW JERSEY (USA)
The Trio Elite Sting Free Adhesive Remover is formulated to offer an advanced solution for individuals requiring stoma care. It utilizes a patented 100% silicone formulation, ensuring a hypoallergenic and sting-free experience. This remover is specifically designed for ease and effectiveness in removing adhesives commonly used in stoma care, such as base plates, wafers, pastes, and hydrocolloid ...
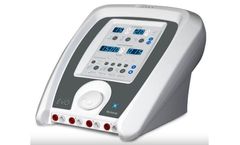
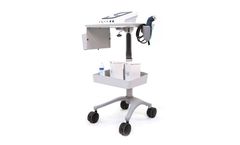
Manufactured by:Tens Units based inLargo, FLORIDA (USA)
The InTENSity CX4 with Therapy Cart is an advanced combination four-channel electrotherapy and ultrasound system offering the practitioner a wide range of treatment options in one user-friendly, ergonomic design. Equipped with a color, touchscreen menu-driven interface intuitively groups and displays clinical protocols, guiding clinicians step-by-step to ensure the ideal therapeutic ...
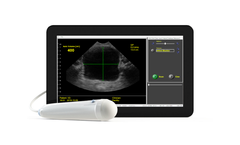
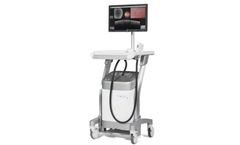
Manufactured by:SeeMore Imaging Canada based inGibsons, BRITISH COLUMBIA (CANADA)
The ViewBladder10 tablet bladder scanner by SeeMore offers a comprehensive solution for non-invasive bladder volume measurement. Featuring multiple operational modes such as Quick Check Mode, the scanner allows fast determination of Post Void Residual Volume without the necessity of manual measurement, making a full bladder easily detectable. The Reference Mode integrates preloaded clinical ...
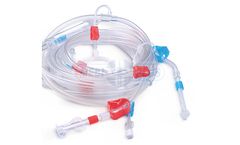
Manufactured by:Shree Umiya Surgical Pvt. Ltd. based inAhmedabad, INDIA
Medical grade PVC tubing for higher bio-compatibility. Advantage of built-in Heparin and Saline line. Extra corporeal blood circuit tubing set, used during dialysis. Low Priming Volume. Easy of use. Customised blood tubing set as per clinic protocol and for different dialysis machines are available. Injection Site : Large finger guard. Drip Chamber : Available Various diameter sizes. Clamp : ...
Manufactured by:Pepex Biomedical, Inc. based inNorcross, GEORGIA (US) (USA)
According to the Centers for Disease Control and Prevention (CDC), Sepsis is the tenth most common cause of death overall in the U.S. Sadly, there is no commercially available test that actually detects the sepsis bacteria and current clinical protocols are simply a process of ...
Manufactured by:Rx Count Corporation based inIrvine, CALIFORNIA (USA)
Meet the easy, accurate way to automatically count tablets and capsules. For any environment where efficiency matters, you can count on the ...
Manufactured by:Richmar based inClayton, MISSOURI (USA)
The TheraTouch EX4 is an advanced four-channel electrotherapy system offering a wide range of treatment options in one user-friendly, ergonomic ...
Manufactured by:Richmar based inClayton, MISSOURI (USA)
The TheraTouch CX2 and Theratouch CX4 are advanced combination two and four-channel electrotherapy and ultrasound systems. Both offer a wide range of treatment options in one user-friendly, ergonomic design. The CX4 offers four channels for stimulation, while the CX2 is a more compact two-channel ...
Manufactured by:Viora based in, NEW YORK (USA)
Want to meet your customers’ needs with advanced light and RF technology? Viora’s V20 Multi-Technology Platform delivers! Viora’s V20 Multi-Technology platform – part of the V-Series – provides treatment solutions for the most requested ...
Manufactured by:ACP - Accelerated Care Plus Corporation based inReno, NEVADA (USA)
The OmniSWD® Shortwave Diathermy System provides electromagnetic energy for healing and heating effects in the body. The thermal setting improves treatment efficiency by 25%. With ACP's integrated, evidence-based clinical protocols, on-screen tuning, and a high efficiency output, the OmniSWD saves therapists valuable time to focus on what matters most - achieving the best outcomes for their ...
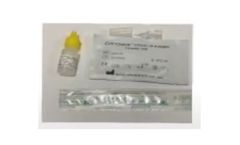
Manufactured by:Spark Mobile Diagnostics based inJamnagar, INDIA
CoV-Check DIRECT Covid-19 Antigen Test is a rapid immunochromatographic assay that utilizes specific monoclonal antibodies to detect Nucleocapsid protein of SARS-CoV-2 virus in nasopharyngeal swab specimens. This test provides a quick and easy to use test to help in the diagnosis of SARS-CoV-2 infection to humans. ...
Manufactured by:ACP - Accelerated Care Plus Corporation based inReno, NEVADA (USA)
The all-new OmniVersa® Multi-Modality Therapy System combines electrotherapy and ultrasound and includes integrated, evidence based clinical protocols and offers ultrasound and a variety of electrical stimulation waveforms to effectively address acute, subacute, and chronic pain presentations for superior pain ...
Manufactured by:Modus Medical Devices based inLondon, ONTARIO (CANADA)
The QUASAR Heavy Duty Respiratory Motion Platform is a programmable breathing and patient motion simulator for the quality assurance of motion-guided radiation therapy systems using any large-scale phantoms up to 45 kg (100 lbs). The Platform HD’s unique multi-directional motion simulation capability enables in-line translation with both the sagittal plane in the SI direction, or at a ...
Manufactured by:Michelson Diagnostics Ltd. (MDL) based inMaidstone, UNITED KINGDOM
VivoSight Dx is indicated for use in the two-dimensional, cross-sectional, real-time imaging of external tissues of the human body. Breakthrough structural and vascular imaging through multi-beam OCT. The VivoSight Dx handheld probe captures a 6 mm x 6 mm scan and produces an image that visualizes 1 mm deep into the dermis. It takes only 15 seconds for a structural scan, and 30 seconds for a ...
Manufactured by:DEKA Dental Lasers based inTampa, FLORIDA (USA)
The DEKA SmartPerio 10W Nd:YAG Laser is a sophisticated piece of dental technology catering to a wide range of dental applications, including periodontics, endodontics, and the treatment of peri-implantitis. This laser operates with a power output of up to 10 watts and frequencies reaching 100 Hz. Its adjustable pulse widths and biostimulation features assist in processes like wound healing and ...
Manufactured by:Modus Medical Devices based inLondon, ONTARIO (CANADA)
Radiation Therapy technologies, such as IGRT, SGRT and respiratory gating, have led to significant advances in patient treatment. The QUASAR™ Respiratory Motion Phantom (pRESP) is a programmable breathing and tumour motion simulator for end-to-end quality assurance on motion-guided radiation therapy systems including CT, LINAC, and PET. The QUASAR Respiratory Motion Phantom has been adopted ...
Manufactured by:Ariana Pharma based inParis, FRANCE
Ariana® has consolidated multiple, publicly available administrative healthcare admissions databases into a locally searchable observational health data resource of patient hospitalization, outpatient and other episodes, comprising in excess of 6B records. ...
by:Soterix Medical Inc based inWoodbridge, NEW JERSEY (USA)
Since 2008, across over 180 published trials, and 15,000 stimulation sessions, the Soterix Medical 1x1 tDCS is the recognized standard in tDCS research. The 1x1 tDCS anchors the 1x1 class of stimulators, with unique features such as RELAX, SmartSCAN, and TrueCurrent with exhaustively validated accessories. The upgraded 1x1 tDCS includes LTE and Adaptive Current to 5 mA. ...
Manufactured by:Indee Labs based inBerkeley, CALIFORNIA (USA)
Hydropore™ is a simple, scalable and efficient way to accelerate the discovery, development and manufacturing of gene-modified cell therapies (GMCTs) such as T cell immunotherapies. This novel, non-viral technology utilizes the power of microfluidic vortex shedding (µVS) to rapidly deliver nucleic acids, proteins and gene-editing complexes to immune cells in a matter of ...