- Home
- Equipment
- north america
- clinical routine diagnosis
Show results for
Refine by
Clinical Routine Diagnosis Equipment Supplied In North America
38 equipment items found
Manufactured by:Clarius Mobile Health Corp based inVancouver, BRITISH COLUMBIA (CANADA)
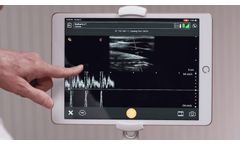
Pulsed Wave Doppler (PWD) allows for accurate measurement and visualization blood flow within vessels. PWD can determine both speed and direction of the flow, and is a requirement for making clinical diagnoses around stenosis and many other cardiovascular ...
Manufactured by:Bardy Diagnostics, Inc. based inBellevue, WASHINGTON (USA)
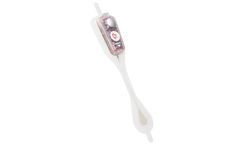
Designed to be placed along the sternum – over the heart – to optimize P-wave signal capture, the CAM patch results in improved ECG resolution, providing more information about heart rhythm that may lead to more clinically-actionable diagnoses. Its unique form factor is designed with comfort and satisfaction in mind, with the aim of improving patient compliance. ...
Manufactured by:Cognoptix Inc. based inMarlborough, MASSACHUSETTS (USA)
Cognoptix’ SAPPHIRE platform is a compact, easy to use, clinical device for in situ patient examination in the comfort of a doctor’s office. The combined platform is currently available in the United States for investigational use only and is intended for clinical evaluation and measurement of beta-amyloid aggregation in anatomically defined regions of the anterior ...
Manufactured by:Jant Pharmacal Corporation based inEncino, CALIFORNIA (USA)
RESPONSE CARDIOVASCULAR Cardiac Markers to Optimize Rapid Clinical Diagnosis Healthcare professionals require a diagnostic testing solution driven by rigorous quality control and the highest standards to accelerate patient diagnosis and throughput. The RAMP®200 Platform provides healthcare professionals with a choice of cardiac markers to optimize rapid clinical diagnosis. Using a compact, ...
Manufactured by:Acteon Group based inMount Laurel, NEW JERSEY (USA)
X-MIND Prime is the latest addition to ACTEON’s CBCT digital imaging product line, now offering a new CEPH option for more clinical applications. Extend the fields of the device for orthodontic analysis, improving diagnosis and treatment planning with high-performance image filtering. X-MIND Prime with CEPH’s patented collimation system allows multiple image size selections, including ...
Manufactured by:Bionote based inBig Lake, MINNESOTA (USA)
The first and only rapid, in-clinic canine cardiac biomarker test, Vcheck's cNT-proBNP test allows for identification of this marker to assess NT-proBNP concentration increases secondary to excessive stretching of heart muscle cells, allowing this marker to be used to assess the severity of underlying heart ...
Manufactured by:Siemens Healthcare GmbH based inErlangen, GERMANY
Siemens Healthineers engineered the compact and powerful ACUSON P500™, a portable ultrasound system to equip clinicians with a powerful lineup of clinical applications right at the bedside. It is your go-to platform to evaluate clinical manifestations, facilitate accurate diagnoses, and to assist procedures in emergency and critical care.The ACUSON P500 also offers an IntraCardiac ...
Manufactured by:United Imaging Healthcare Co., Ltd. based inHouston, TEXAS (USA)
The uDR 780i uses United Imaging's innovative, wireless, flat-panel detector design and provides ultra-high resolution in general radiography for daily clinical ...
Manufactured by:SonoScape Medical Corp. based inShenzhen, CHINA
SonoScape’s laptop styled color Doppler ultrasound system, the X5, revolutionized the ultrasound market. Designed as the go-to ultrasound in any setting at the bedside or aboard such as in an ambulance thanks to its light weight, small size and impressive durability. Packed with crystal clear imaging capabilities and advanced applications inside this small laptop system makes it the ideal ...
Manufactured by:MagQu LLC USA based inSurprise, ARIZONA (USA)
The magnetic immunoassay analyzer (Model: XacPro-S) created by MagQu Co., Ltd. is used to measure the change in the ac magnetic susceptibility of a sample over time. If a sample is mixed with our IMR reagent, the concentration of bio-molecules can be measured in correlation with the change in the ac magnetic susceptibility of the mixture. XacPro-S can be applied to molecular detection not only ...
Manufactured by:Biochemazone based inLeduc, ALBERTA (CANADA)
It consists of Amino acids, Minerals, and Metabolites, Other compositions and customer’s specified configurations can be delivered upon request. Stabilizer, pH Value, Pack Size, and Contents of Simulated ECCRINE PERSPIRATION are customizable. Transportation and storage: Transportation at room temperature, storage at 4 ?. For Longterm Storage Freeze it at -20 ?; We provide shipping ...
by:LightDeck Diagnostics based inBoulder, COLORADO (USA)
We are developing quantitative point-of-care, multiplex tests for hormones in ...
Manufactured by:United Imaging Healthcare Co., Ltd. based inHouston, TEXAS (USA)
The uDR 596i is a revolutionary, automated, floor-mounted, high-resolution DR system with wireless, flat-panel ...
Manufactured by:Lexogen GmbH based inVienna, AUSTRIA
Biases in RNA Sequencing: RNA sequencing (RNA-Seq) workflows comprise RNA purification, library generation, the sequencing itself, and the evaluation of the sequenced fragments. The initial steps impose biases for which the data processing algorithms try to compensate afterwards. Key tasks for data evaluation algorithms are the concordant assignment of fragments to the transcript variants, ...
Manufactured by:Bio Molecular Systems based inUpper Coomera, AUSTRALIA
The world’s first magnetic induction cycler is now a registered medical device with CE-IVD and TGA approval. Mic IVD is manufactured under an ISO 13485:2016 Quality Management System. ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
Maxim's FDA Approved HIV-1 Urine EIA test is an enzyme immunoassay for the in-vitro detection of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) in urine. The test is intended for use in professional laboratory settings as an aid in clinical diagnosis of HIV infection. Before a determination of HIV-1 status can be made, specimens that are repeatedly reactive using this test should be ...
Manufactured by:Pacific Edge Ltd based inDunedin,, NEW ZEALAND
Cxcolorectal is a prognostic gene signature for patients diagnosed with stage II or stage III colorectal cancer. The test predicts the aggressiveness of the tumour, allowing physicians to make the best decision regarding treatment for the ...
Manufactured by:Avioq, Inc. based inResearch Triangle Park, NORTH CAROLINA (USA)
The Avioq HTLV-I/II Microelisa System is intended as a screen for donated blood and organs to prevent transmission of HTLV-I and HTLV-II to recipients of cellular blood components and as an aid in clinical diagnosis of HTLV-I or HTLV-II infection and related ...
Manufactured by:Wellue based inDiamond Bar, CALIFORNIA (USA)
LONG-TERM ECG TRACKING: Many heart diseases are sporadic, and patients are hard to find abnormalities when they go to the hospital for an electrocardiogram. Because an electrocardiogram time is too short to identify paroxysmal atrial flutter sometimes. A 24-hour dynamic electrocardiogram is commonly used in clinical to assist diagnosis. The Wellue 12-Channel Holter monitor not only can discover ...
Manufactured by:Infitek Co., Ltd. based inJinan, CHINA
As a necessary choice for quantitative analysis of molecular biology, real-time PCR system has been widely used in various fields such as scientific research, clinical detection and diagnosis, quality and safety testing, and forensic ...