- Home
- Equipment
- north america
- clinical trial for success
Refine by
Clinical Trial For Success Equipment Supplied In North America
39 equipment items found
Manufactured by:Mevion Medical Systems based inLittleton, MASSACHUSETTS (USA)
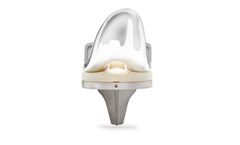
The MEVION S250mx™ is a scalable proton therapy solution with two, three or four room options. Because of Mevion’s unique core technology, each treatment room is fully independent. This gives you the flexibility to add rooms as patient volume grows, allowing you to adapt to increasing demand, minimize financial risk, and maximize your clinical and operational ...
Manufactured by:Merit Medical Systems based inSouth Jordan, UTAH (USA)
A decade – the time it took to build a superior guide wire and to rival competing greatness. The Merit SplashWire combines optimum lubricity, exceptional torque response, and enhanced visibility, helping you achieve the successful outcomes you expect, even in the most complex ...
Manufactured by:Diagnosys LLC based inLowell, MASSACHUSETTS (USA)
The DiagnosysFST®, Full-field Stimulus Threshold, module uses the ColorDome™or ColorFlash™ stimulator. It measures the sensitivity of the visual field by testingfor the lowest luminance flash which elicits a visual sensation perceived by thesubject. The test is run on either dark- or light-adapted patients for one or botheyes in an automated routine to measure a reliable ...
Manufactured by:Verge Genomics based inSouth San Francisco, CALIFORNIA (USA)
The breadth and depth of our pipeline demonstrates the power of the ConVERGEMplatform. All pipeline programs have been discovered and developed on our proprietary platform. ...
Manufactured by:Centric Medical, LLC based inHuntley, ILLINOIS (USA)
The Saturn External Fixation System is composed of rings, struts, threaded rods, pins, wires and connectors. It is intended to be used as a means to stabilize bone segments for indications within the foot and ...
Manufactured by:Ortho Development Corporation based inDraper, UTAH (USA)
The Ovation Hip System is designed with optimal refinements to the best clinically proven M/L taper stems, providing 3-points of fixation and excellent rotational stability. Ovation and Ovation Tribute have simple, intuitive instrumentation to provide intraoperative ...
Manufactured by:Ortho Development Corporation based inDraper, UTAH (USA)
The Balanced Knee System offers proven and reproducible results and has over 20 years of successful clinical ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The PhysioStim device provides a safe, non-invasive option for treating fractures that are difficult to heal. With an overall clinical success rate of 80 percent (up to 88 percent for fracture gaps less than 3mm), PhysioStim devices have high success rates for treating nonunion fractures. The device assists in fracture healing by delivering a pulsed electromagnetic field (PEMF) signal to the ...
Manufactured by:Catalent, Inc based inSomerset, NEW JERSEY (USA)
AAV, or adeno-associated virus, is considered the gold standard for in vivo gene delivery transfer and has become the leading viral vector choice for gene therapies today. As one of the field’s most effective delivery tools, AAV has shown strong clinical success and recent improvements in related technology and system approaches have made AAV production an efficient means to meet clinical ...
Manufactured by:Catalent, Inc based inSomerset, NEW JERSEY (USA)
AAV, or adeno-associated virus, is considered the gold standard for in vivo gene delivery transfer and has become the leading viral vector choice for gene therapies today. As one of the field’s most effective delivery tools, AAV has shown strong clinical success and recent improvements in related technology and system approaches have made AAV production an efficient means to meet clinical ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
The collarless, polished, double-taper design concept used in the CPT Hip System has proven itself clinically in more than 25 years of use.¹ This distinctive design philosophy is based on the use of natural compressive forces to help ensure that the implant is firmly seated and wedged within the cement mantle. The CPT System, in cobalt-chromium² and stainless steel,3,4 has achieved ...
Manufactured by:Avalon Medical based inStillwater, MINNESOTA (USA)
MitralSeal™ was designed as the first practical and curative treatment for dogs with severe mitral valve regurgitation, otherwise known as Canine Mitral Valve Disease. MitralSeal is an artificial heart valve designed for transcatheter delivery using a minimally-invasive approach into a beating ...
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
The Quadra-P System offers a complete set of stems developed preserving the characteristics important to the clinical success of Quadra-H while incorporating proven innovative key features to address the modern challenges in ...
Manufactured by:Cabaletta Bio, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
In addition to our work on autoimmune disease treatments, we are investigating the viability of CAART as an adjunct in cases where the immune system produces antibodies against current therapies (rather than against the patient’s cells). We believe our approach has the potential to destroy those antibodies by ablating B cells, similar to the method we have used to combat autoimmune disease. ...
Manufactured by:MicroPort Scientific Corporation based inShanghai, CHINA
The Evolution® medial-pivot knee system is designed to answer the limitations of traditional implants by delivering superior flexion stability, anatomic motion, and wear–limiting design ...
Manufactured by:Microport Orthopedics Inc. based inArlington, TENNESSEE (USA)
The Evolution® medial-pivot knee system is designed to answer the limitations of traditional implants by delivering superior flexion stability, anatomic motion, and wear–limiting design characteristics. The Evolution® medial-pivot knee system with BioFoam® cancellous titanium on the tibial implant is the next generation in cementless fixation. Alongside the medial-pivot knee ...
Manufactured by:Keystone Dental based inBurlington, MASSACHUSETTS (USA)
The Genesis Implant System pioneered implant anodization, imitating the natural pink hue of soft tissue and the characteristics of bone. Backed by a decade of science, the Genesis Implant System achieves predictable aesthetics and long-term clinical success. This is a complete system for all patients and indications, including immediate implant placement, temporization, and more conservative ...
Manufactured by:Innovative Medical Products, Inc. based inPlainville, CONNECTICUT (USA)
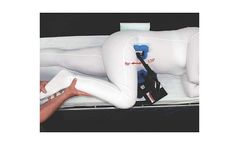
The original hip positioner, our time-tested surgical positioning device for hip surgeries. The latest generation of the McGuire Pelvic Positioner, which was IMP's original lateral positioner for THA. The McGuire was one of the first hip positioners with over 25 years of clinical success. With removable and independently adjustable anterior arms, it allows for more accurate contact with the iliac ...
Manufactured by:TransLite LLC based inSugar Land, TEXAS (USA)
The all-new PEDI2 is the perfect solution for eliminating multiple sticks in children during venipuncture procedures. It's the world’s best vein access device for use in Pediatric medicine, replacing the Veinlite PEDI model. It is compact and versatile, perfect for children of all ages, from infants to adolescents. PEDI2 outperforms the original PEDI in every way, adding new features like ...
Manufactured by:NuVasive, Inc. based inSan Diego, CALIFORNIA (USA)
The most clinically effective technology in the cTDR procedure segment*. The Simplify Cervical Artificial Disc is backed by an FDA investigational device exemption (IDE) study, and showed superiority to ACDF.3. The Simplify Disc is designed to offer surgeons best-in-class capabilities for cTDR across key performance functions—anatomic, physiologic motion, and radiologic ...