- Home
- Equipment
- north america
- covid antigen
Refine by
Covid Antigen Equipment Supplied In North America
16 equipment items found
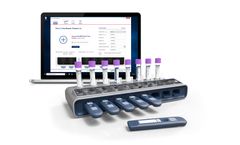
Manufactured by:Ellume Limited based inEast Brisbane, AUSTRALIA
In partnership with global diagnostics company QIAGEN®, Ellume has developed two rapid COVID-19 tests: the QIAreach™ SARS-CoV-2 Antigen Test**** for active infections and the QIAreach™ Anti-SARS-CoV-2 Total Test** for past infections. Both tests are now available in the U.S. The tests are run on the eHub platform, an easy-to-use and portable ...
Manufactured by:Ellume Limited based inEast Brisbane, AUSTRALIA
Two tests can be performed at the same time, with simple, animated on-screen instructions and rapid results in 15 minutes. The COVID Antigen test (for the detection of active COVID-19 infections) is currently available***. The COVID Serology test (for the detection of total antibodies to SARS-CoV-2) is pending regulatory ...
Manufactured by:Hemex Health based inPortland, OREGON (USA)
Hemex Health is working to empower the frontlines of healthcare with our inexpensive, portable, and easy-to-use diagnostic device - Gazelle®. Gazelle provides affordable, accurate testing to new populations at sites where it is needed most. Hemex designs products for the real world by listening to the needs of providers including those located in remote and challenging settings. Initial ...
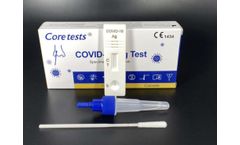
Manufactured by:Coretests, Inc. based inCarlsbad, CALIFORNIA (USA)
COVID-19 Ag Test is the chromatographic assay test used for qualitative detection of the COVID-19 nucleoprotein antigen in nasal swab specimen from, suspected patients may infected COVID-19. It is intended to be used in self-testing., Specimen:Nasal swab, Detection time at15 minutes, No need for extra equipment, High accuracy, ...
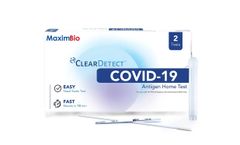
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
The MaximBio ClearDetect™ COVID-19 Antigen Home Test is an antigen test that allows you to detect proteins from the virus that causes COVID-19. Made easy - An over-the-counter, no prescription needed nasal swab test you can do anywhere & anytime. Know your COVID-19 status in as little as 15 minutes! ...
by:LightDeck Diagnostics based inBoulder, COLORADO (USA)
We are developing a test to detect current infection by the SARS-CoV-2 ...
Manufactured by:ACON Laboratories, Inc. based inSan Diego, CALIFORNIA (USA)
Critical Information When & Where You Need It; The Flowflex™ COVID-19 Antigen Home Test is all you need to determine your family’s Covid-19 status, whether symptoms are present or not. Can be used on children as young as 2 years old. Get the convenience of ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
A rapid immunoassay for the simultaneous direct detection and differential diagnosis of SARS-CoV-2, Influenza Type A and Type B antigen from anterior nasal (AN) or nasopharyngeal (NP) swab specimens. Kit includes extraction buffer vials and flocked nasopharyngeal (NP) swabs for superior specimen collection and patient comfort, and provides a visual read-out in 15 minutes with no equipment needed! ...
Manufactured by:Spark Mobile Diagnostics based inJamnagar, INDIA
CoV-Check DIRECT Covid-19 Antigen Test is a rapid immunochromatographic assay that utilizes specific monoclonal antibodies to detect Nucleocapsid protein of SARS-CoV-2 virus in nasopharyngeal swab specimens. This test provides a quick and easy to use test to help in the diagnosis of SARS-CoV-2 infection to humans. ...
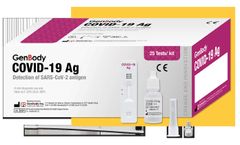
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
Intended use: iHealth® COVID-19 Antigen Rapid Test is a lateral flow assay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 15 years or older with symptoms of ...
Manufactured by:Response Biomedical Corp. based inVancouver, BRITISH COLUMBIA (CANADA)
Our all-in-one RAMP® COVID-19 Antigen kit offers on the spot actionable results you can trust. Combined with our RAMP® 200 instrument, we offer rapid triage of nasal swab samples in 15 minutes. ...
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
Intended Use: INDICAID™ COVID-19 Rapid Antigen Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARSCoV-2 in direct anterior nasal swab specimens from individuals who are suspected of COVID-19 by their healthcare provider within the first five (5) days of symptom ...
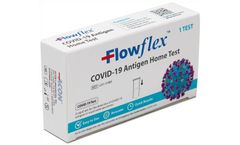
Manufactured by:Mountain Horse Solution based inUnion City, TENNESSEE (USA)
The Flowflex COVID-19 Antigen Home Test is a rapid test for the detection of SARS-CoV-2. The lateral flow chromatographic immunoassay is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in anterior nasal swab specimens directly from individuals within 7 days of symptom onset or without symptoms or other ...
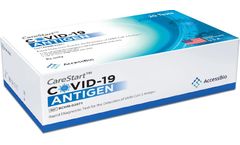
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
Self Test for SARS-CoV-2 Antigen Detection. The CareStart™ COVID-19 Antigen Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigens from SARS-CoV-2. Fast and easy to self-test at anywhere. Easy to interpret the results using mobile application. ...
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
An immunochromatographic rapid diagnostic test (RDT) intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) or anteriornasal (AN) swab specimens. Authorized for use under an Emergency Use Authorization (EUA) and for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare ...