- Home
- Equipment
- north america
- covid virus
Show results for
Refine by
Covid Virus Equipment Supplied In North America
14 equipment items found
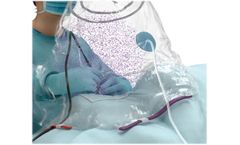
Manufactured by:Grace Medical based inMemphis, TENNESSEE (USA)
Recent research has shown there is a heightened risk of exposure to SARS-CoV-2 virus (COVID-19) during middle ear or mastoid procedures. Grace Medical recognized the need to develop protective gear to mitigate aerosol dispersion of potentially contaminated particles and protect healthcare professionals. The OtoShield Protective Barrier Drape uses a self-sealing ...
Manufactured by:ImmunoPrecise Antibodies Ltd. based inVictoria, BRITISH COLUMBIA (CANADA)
In addition, the encoded protein is a functional receptor for the spike glycoprotein of the human coronavirus HCoV-NL63 and the human severe acute respiratory syndrome coronaviruses, SARS-CoV and SARS-CoV-2 (COVID-19 ...
Manufactured by:ImmunoPrecise Antibodies Ltd. based inVictoria, BRITISH COLUMBIA (CANADA)
In addition, the encoded protein is a functional receptor for the spike glycoprotein of the human coronavirus HCoV-NL63 and the human severe acute respiratory syndrome coronaviruses, SARS-CoV and SARS-CoV-2 (COVID-19 ...
Manufactured by:America Diagnostics, Inc. based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
This product is for in vitro diagnostic use, following guidance from the FDA for Emergency Use Authorizations of tests submitted for approval on March 16, 2020. This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
Manufactured by:America Diagnostics, Inc. based inSan Diego, CA 92126 USA, CALIFORNIA (USA)
This product is for in vitro diagnostic use, following guidance from the FDA for Emergency Use Authorizations of tests submitted for approval on March 16, 2020. This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
Manufactured by:Anavasi Diagnostics based inSeattle, WASHINGTON (USA)
The AscencioDx COVID-19 Molecular Detector is a rapid, highly accurate, easy-to-use and affordable clinical diagnostic platform used for performing molecular analysis of samples collected from patients in point-of-care (POC) settings utilizing the proprietary AscencioDx COVID-19 Test Kits. ...
Manufactured by:Elabscience based inHouston, TEXAS (USA)
COVID-19 (Corona Virus Disease) is an infectious disease caused by the most recently discovered coronavirus. COVID-19 IgG/IgM Rapid Test ( Serum/Plasma/Whole Blood) is a rapid chromatographic immunoassay for the qualitative of IgG and IgM antibodies to COVID-19 in human serum, plasma or whole ...
Distributed by:Labex Canada Inc. based in, ONTARIO (CANADA)
The COVID-19 Vaccine IgG Rapid Test is a lateral flow immunoassay intended for the qualitative detection of antibodies binding to the Receptor Binding Domain (RBD) of the spike protein of the SARS-CoV-2 virus or COVID-19 vaccine in human serum, plasma or whole blood. The kit is intended for use as an aid in identifying individuals with an ...
Manufactured by:Lucira Health - Pfizer based inEmeryville, CALIFORNIA (USA)
The Lucira Check It is a molecular or nucleic acid amplification test (NAAT) that provides accurate, at-home results for the detection of the COVID-19 virus. Our tests have an analytical sensitivity (ability to detect the SARS-CoV-2 virus) that is comparable to some of the best performing PCR tests in clinical settings and high complexity ...
by:Diasorin based inSALUGGIA, ITALY
DiaSorin strives to improve health-care around the globe through the development of innovative diagnostic solutions that can lead to better patient outcomes and provide economic benefits to the healthcare system. Thanks to its team of dedicated scientists DiaSorin has been able to promptly deliver diagnostic solutions to manage the COVID-19 pandemic ...
Manufactured by:OPTI Medical Systems, Inc. based inRoswell, GEORGIA (US) (USA)
OPTI Medical Systems has received US FDA Emergency Use Authorization and CE mark for its OPTI SARS-CoV-2 RT-PCR Test for detection of the virus causing COVID-19. The OPTI SARS-CoV-2 RT-PCR Test is based on real-time reverse transcription polymerase chain reaction (RT-PCR), which provides detection of the viral RNA in the sample. The test can be used on both ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Ebselen (SPI-1005), a glutathione peroxidase mimetic, is a potent voltage-dependent calcium channel (VDCC) blocker[1][2]. Ebselen potently inhibits Mpro (IC50=0.67 μM) and COVID-19 virus (EC50=4.67 μM)[3].Ebselen is an inhibitor of HIV-1 capsid CTD dimerization. Ebselen, an organoselenium compound, can permeate the blood-brain barrier and has anti-inflammatory, ...
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
COVID-19 is caused by the SARS-CoV-2 virus which is a new virus in humans causing a contagious respiratory illness. COVID-19 can present with a mild to severe illness, although some people infected with COVID-19 may have no symptoms at all. Older adults and people of any age who have underlying medical conditions ...
Manufactured by:Metrex Research, LLC. based inOrange, CALIFORNIA (USA)
Durable, non-woven towelettes that offer quick, easy-to-use, time-saving convenience killing organisms in only 3 minutes. For use on hard non-porous surfaces of non-invasive medical ...