- Home
- Equipment
- north america
- drug therapy
Show results for
Refine by
Drug Therapy Equipment Supplied In North America
39 equipment items found
Manufactured by:Khloris Biosciences, Inc. based inMountain View, CALIFORNIA (USA)
By developing cardiomyocytes directly from patient-derived iPSCs, we create a non-invasive, personalized platform to directly observe the effects of drug therapies on heart ...
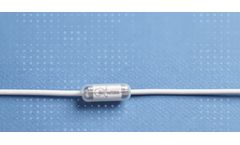
Manufactured by:Pepex Biomedical, Inc. based inNorcross, GEORGIA (US) (USA)
Inline 3 Electrode Sensor Module Used in Drug Therapy Monitoring & intervention. CCM 3E Inline 3 Electrode Sensor Module for Trend Monitoring for In-Hospital Diagnostics. ...
Manufactured by:Apollo Endosurgery, Inc. based inAustin, TEXAS (USA)
ORBERA fills the gap between the non-surgical weight loss segment and the weight loss surgery segment, allowing you to do more for your patients who require more than drug therapy and dietary advice. The comprehensive, two-part program takes a holistic approach to weight loss, combining the use of an intragastric balloon with an individually tailored support ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines is developing a GeneVav prime/VesiculoVax™ boost therapeutic vaccine that targets all HCV genotypes for use as an adjunct to drug ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has also developed a GeneVax prime/VesiculoVax boost therapeutic vaccine that targets all eight HBV genotypes for use as an adjunct to drug ...
Manufactured by:Setpoint Medical Corporation based inValencia, CALIFORNIA (USA)
SetPoint Medical created a therapy to offer patients and providers a better alternative for the treatment of chronic inflammatory diseases with potentially less risk and lower cost than drug therapy. SetPoint Medical’s bioelectronic system is free of routine injections or pills and may eliminate the risks and substantial cost associated ...
Manufactured by:Advanced Accelerator Applications SA based inRueil-Malmaison Cedex, FRANCE
AAA brand names for [18F]-choline (FCH). ...
Manufactured by:Advanced Accelerator Applications SA based inRueil-Malmaison Cedex, FRANCE
AAA brand names for 6-fluoro-[18F]-L-DOPA, a DOPA analog. Neurology, ...
Manufactured by:Pluristem Therapeutics Inc. based inHaifa, ISRAEL
Our convenient, automated thawing device, for use by clinicians at point of care, offers a reliable and simple thawing method that can safeguard the integrity of the supply chain. This is key to maintaining the potency and consistency of a cell product, because, unlike many traditional drugs, cell therapies must be thawed before being administered to a patient. ...
Manufactured by:Ellex Inc. based inMinneapolis, MINNESOTA (USA)
The Ellex Solo™ is an advanced SLT (selective laser trabeculoplasty) laser which allows you to perform quick and highly accurate glaucoma laser treatment customized to each individual patient’s response. SLT is gentle and non-invasive, which means you can stimulate healthy trabecular meshwork cell regeneration and manage IOP without producing burn scar tissue. ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
Drug-free success that works. Rapport vacuum therapy devices have been developed as user-friendly, effective treatments for erectile ...
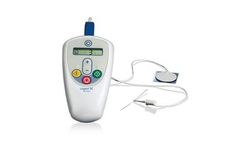
Manufactured by:Laborie Medical Technologies Corp. based inPortsmouth, NEW HAMPSHIRE (USA)
Urgent PC delivers percutaneous tibial nerve stimulation (PTNS) to treat patients with overactive bladder and the associated symptoms of urinary urgency, urinary frequency and urge ...
Manufactured by:Adaptive Biotechnologies based inSeattle, WASHINGTON (USA)
We are currently leveraging our TCR discovery capabilities to enable commercialization of novel therapies by collaborators. In the future, we may explore expanding our end-to-end capabilities for the development of cellular therapies and vaccines. TruTCR is summarized in the figure ...
Manufactured by:Reglagene Holding, Inc. based inTucson, ARIZONA (USA)
Only 4 of the 217 FDA-approved oncology medicines are indicated for life-threatening brain cancers. The most recent approval was in 2009, but this medicine did not extend life expectancy. Patients deserve better therapies that extend life and improve quality of life. The Reglagene team is developing a first-in-class blood–brain barrier (BBB) penetrant medicine that effectively fights brain ...
Manufactured by:ACI Medical, LLC based inSan Marcos, CALIFORNIA (USA)
The APG® Air Plethysmograph is a non-invasive diagnostic tool that quantifies the physiological components of chronic venous disease including: Chronic obstruction; Valvular reflux; Calf muscle pump function; Venous hypertension. The APG® Air Plethysmograph is the only system that measures true blood volume changes in milliliters and blood flow in milliliters per second. The APG® ...
by:Vor Biopharma based inCambridge, MASSACHUSETTS (USA)
VOR33 is our lead eHSC product candidate designed to replace the standard of care in transplant settings. Once the VOR33 cells have engrafted, we believe that patients can be treated with anti-CD33 therapies, such as Mylotarg or VCAR33, with limited on-target toxicity, leading to durable anti-tumor activity and potential cures. In preclinical studies, we have observed that the removal of CD33 ...
Manufactured by:Restech based inHouston, TEXAS (USA)
The first and only medical sensor able to monitor real-time, continuous pH in both liquid and aerosolized states, revealing reflux-related etiology of presenting symptoms (e.g. chronic cough, hoarseness, throat clearing, and globus). The sensor is unique in its ability to accurately function anywhere in the aerodigestive tract—from the esophagus to the nasopharynx—reporting ...
Manufactured by:Chronolife SAS based inParis, FRANCE
Collecting real-life data with a certified, comfortable, and 100% washable connected medical device which improves patient compliance. Keesense is a connected medical device (CE Class IIa) in the form of a t-shirt that allows real-life measurement of multiple physiological parameters. All sensors and electronics are fully integrated into the t-shirt, therefore making it very comfortable to wear ...
Manufactured by:Chrono-log Corporation based inHavertown, PENNSYLVANIA (USA)
The CHRONO-LOG® WBA Systems are available in two (2) or four (4) channel configurations … providing testing of Platelet Function in Whole Blood samples utilizing either Reusable or Disposable Probes. ...
Manufactured by:Sterlitech Corporation based inAuburn, WASHINGTON (USA)
Sterlitech Polyethersulfone (PES) membrane is a hydrophilic membrane constructed from pure polyethersulfone polymer membrane. PES membrane filters are designed to remove particulates during general filtration. Their low protein and drug binding characteristics also make polyethersulfone (PES) membrane disc filters ideally suited for use in life science ...