- Home
- Equipment
- north america
- fusion surgery
Show results for
Refine by
Fusion Surgery Equipment Supplied In North America
22 equipment items found
Manufactured by:Enovis based inLewisville, TEXAS (USA)
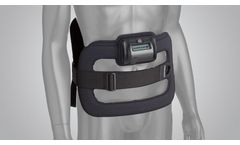
CMF Spinalogic is a portable, battery-powered, micro-controlled, noninvasive bone growth stimulator indicated as an adjunct electromagnetic treatment to primary lumbar spinal fusion surgery for one or two ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The SpinalStim device is FDA approved to be used after spinal fusion surgery or to be used to treat a failed fusion from a previous surgery. The devices stimulate the natural healing process of bone by sending low-level pulses of electromagnetic energy to the injury or fusion site. The device has an overall ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
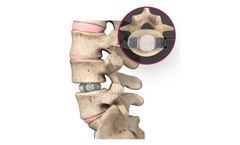
The pedicle screw, which is sometimes used as an adjunct to spinal fusion surgery, provides a means of gripping a spinal segment. The screws themselves do not fixate the spinal segment, but act as firm anchor points that can then be connected with a rod. The screws are placed by the surgeon into two or three consecutive spine segments (e.g. lumbar segment 3 and ...
Manufactured by:Alevio, LLC based inBirmingham, ALABAMA (USA)
If non-surgical treatments do not provide relief, you may be a candidate for minimally invasive SI joint fusion surgery. This minimally invasive surgical procedure eliminates motion by stabilizing and fusing the SI joint. The procedure is done through a posterior approach with one or two small incisions on your buttock. ...
Manufactured by:Spineology Inc. based inSt. Paul, MINNESOTA (USA)
The Duo Ti system represents the latest advancements in lateral interbody fusion technology. The system minimizes the amount and duration of retraction on the neural structures and surrounding soft tissues, both of which have been linked to neurologic deficits following lateral interbody fusion surgery.1, ...
Manufactured by:Ankasa Regenerative Therapeutics based inLa Jolla, CALIFORNIA (USA)
Ankasa is the first to produce human Wnt proteins in a manufacturing setting suitable for use in humans. Our first clinical approach will be the demonstration of improved outcomes after spinal fusion surgeries. Our product has expansion opportunities to other bone reconstructive procedures including osseointegration of dental and orthopedic implants (knee and hip ...
Manufactured by:Arrowhead Medical Device Technologies, LLC based inCollierville, TENNESSEE (USA)
Firmly grasps the ARROW-LOK implant preventing both rotation and translation to ensure the implant is placed securely in the broached pilot hole. Cannulated to hold the implant securely. Provides a positive stop to prevent the implant from translating when placing the intermediate phalanx over the implant. Able to grasp the implant at the position determined by the surgeon to place the implant in ...
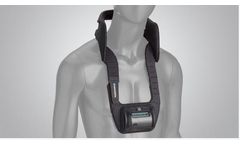
Manufactured by:Theragen based inManassas, VIRGINIA (USA)
Introducing the newest, most compact1 way to deliver FDA-approved lumbar spine fusion therapy2 — now with a companion digital experience that lets you see your progress in ...
Manufactured by:Kuros Biosciences A.G. based inSchlieren, SWITZERLAND
MagnetOs isn’t like other bone grafts. It grows bone even in soft tissue thanks to our unique NeedleGrip™ surface technology which provides traction for our body’s vitally important ‘pro-healing’ immune cells (M2 macrophages).*†6,7 This in turn, unlocks previously untapped potential to stimulate stem cells – and form new bone throughout the graft.*8-10 ...
Manufactured by:Advin Urology based inAhmedabad, INDIA
ADVIN Vessel Sealing System (SAFESEAL +) is a next-generation in energy-based vessel sealing technology. It presents an improved and safe vessel sealing procedure in the medical sector. It is known as Vessel Sealing Machine, Vessel Sealing Diathermy Unit, Monoseal Vessel Sealing System, High-Frequency Diathermy Machine With Vessel Sealing Unit, Laparoscopic Vessel Sealing System And Vessel ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
Cervical spinal fusion (arthrodesis) is a surgery that joins selected bones in the neck (cervical spine). Cervical fusion is sometimes used to treat acute injuries, such as fractures and dislocations, and to treat mechanical or structural problems causing disabling pain (such as from cancer or infection). In many instances, cervical ...
Manufactured by:ChoiceSpine LLC based inKnoxville, TENNESSEE (USA)
Hawkeye TI is changing the game for cervical corpectomy cases. With its 510(k) cervical clearing, 8° lordosis, and multiple footprints corpectomy cases are now more efficient than ever. Hawkeye TI is getting surgeons and patients out of the operating room and on the road to recovery faster and more ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc is designed for use using a lateral traspsoas or retroperitoneal (i.e., anterolateral) surgical approach to access disc space. Access through the annulus can be achieved using the PerQdisc™ Disc Access System (Figure 3). Once annular access is achieved, a nuclectomy (removal of nucleus material) is performed through the disc access using various tools to remove most of the ...
by:PainTEQ based inTampa, FLORIDA (USA)
The LinQ SI Joint Stabilization System provides SI joint dysfunction patients with a minimally invasive option to combat pain. After a thorough diagnostic process, physicians may help alleviate, and in many cases eliminate, chronic pain by placing a single LinQ allograft into the SI joint. This single implant may help patients immediately regain joint stability – and with its ...
Manufactured by:Orthofix Medical Inc. based inLewisville, TEXAS (USA)
The CervicalStim device is the only bone growth stimulation therapy approved by the FDA as a noninvasive, adjunctive treatment option for cervical fusion in patients at high-risk for non-fusion. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing at a ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc Implant Delivery Device (Figure 4) is then inserted through the Access Cannula into the enucleated disc space. Contrast is injected into the inner chamber of the implant. This aids in visualization as well as provides real-time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a curable, polymer containing barium sulfate. The ...
Manufactured by:Mesoblast Ltd. based inMelbourne, AUSTRALIA
MPC-25-Osteo for spinal fusion is a proprietary Phase 3-ready product candidate. All doses of MPC-25-Osteo for the treatment of spinal fusion consist of 25 million MPCs delivered on a collagen ceramic carrier material into the disc space with stabilizing ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The SST PerQdisc Nucleus Replacement System is intended to replace the nucleus pulposus of the intervertebral disc in the L1-S1 spinal region in patients with single-level discogenic pain. The patient may have single or multi-level degenerative disc changes but the discogenic pain must be limited to a single level. The patient must be skeletally mature, at least 21 years of age, presenting with ...
Manufactured by:Alevio, LLC based inBirmingham, ALABAMA (USA)
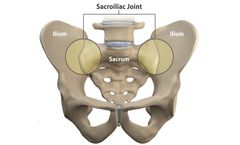
The sacroiliac (SI) joint is located between the sacrum and ilium and connects the spine to the pelvis. The SI joint is the largest joint in the spine and allows the transfer of forces between the lumbar spine and the lower body. If the SI joint is injured or degenerates over time, it may cause chronic pain. ...
by:Empirical Spine, Inc. based inSan Carlos, CALIFORNIA (USA)
LimiFlex is intended to provide segmental stabilization following decompression for grade 1 lumbar degenerative spondylolisthesis with spinal stenosis. LimiFlex is implanted through a dorsal approach, typically through the same incision used for the surgical ...