- Home
- Equipment
- north america
- implant fit
Show results for
Refine by
Implant Fit Equipment Supplied In North America
16 equipment items found
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
The OrthoPediatrics Wrist Fusion System is an extension of the PediFrag system, and is indicated for wrist arthrodesis and fractures of other small bones in children, adolescents, and small stature adults. The system consists of two implant length offerings, to fit patients both with and without a proximal row carpectomy (PRC). Bending templates are included in ...
Manufactured by:ARCH Medical Solutions Corp. based inScarborough, MAINE (USA)
The Arcam EBM Q10plus is particularly ideal for the production of high volume, press fit implants with advanced trabecular structures as derived from CT scans of individual ...
Manufactured by:Spine Wave, Inc. based inShelton, CONNECTICUT (USA)
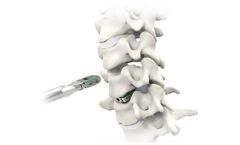
The Velocity Expandable Interbody Device is a composite, expandable implant that is designed for ease of insertion, optimal implant to patient fit, maximized indirect decompression, and maximum pre and post bone ...
Manufactured by:Spine Wave, Inc. based inShelton, CONNECTICUT (USA)
Flexible Offering, Calibrated Design. The Abacus® Lateral Spacer System is available in multiple sizes with a biconvex implant shape designed to provide conformance to patient anatomy/pathology. The Abacus® Lateral Spacer System is intended to be introduced to the disc space via the lateral ...
Manufactured by:Spinal Elements, Inc. based inCarlsbad, CALIFORNIA (USA)
Simple Reproducible Procedure. Single Tray and one-size fit all implant compliment a simple, efficient, and straight-forward procedure. PEEK fixation strap placed with a proprietary curved drill provides circumferential cortical fixation through the lamina, pedicle, and IAP/SAP of the facets. ...
Manufactured by:Bioventus Inc. based inDurham, NORTH CAROLINA (USA)
Agili-C is a cell-free, off-the-shelf implant for use in cartilage and osteochondral defects in traumatic and osteoarthritic joints. The implant is a porous, biocompatible, and resorbable bi-phasic scaffold, consisting of interconnected natural inorganic calcium carbonate ...
Manufactured by:Smith & Nephew plc based in, UNITED KINGDOM
The ANTHEM◊ Total Knee System was designed to provide an advanced and anatomically fit prosthesis and instrumentation ...
Manufactured by:Arrowhead Medical Device Technologies, LLC based inCollierville, TENNESSEE (USA)
Commit to trying ARROW-LOK™ on at least five patients while closely following the recommended technique. As simple as the ARROW-LOK™ is and uncomplicated the operative technique may be, please expect to take at least five cases to become comfortable with the product, the operative technique and how the design interacts with the anatomy. Five (5) cases will give the surgeon the ...
Manufactured by:Enovis based inLewisville, TEXAS (USA)
Superior Loading: A shortened hip stem must derive stable loading from it superior regions or risk failure. The Revelation microMAX stem features proximal geometry that maximizes stability and provides physiologic load transfer that no other short stem can ...
Manufactured by:Atlas Spine, Inc. based inJupiter, FLORIDA (USA)
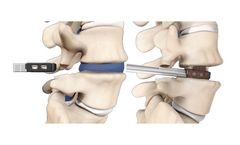
The HiJAK AC Spacer System, the FIRST expandable cervical interbody with ADJUSTABLE HEIGHT and LORDOSIS, was designed for the surgeon who recognizes the importance of lordotic restoration and sagittal balance of the cervical ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc is designed for use using a lateral traspsoas or retroperitoneal (i.e., anterolateral) surgical approach to access disc space. Access through the annulus can be achieved using the PerQdisc™ Disc Access System (Figure 3). Once annular access is achieved, a nuclectomy (removal of nucleus material) is performed through the disc access using various tools to remove most of the ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc Implant Delivery Device (Figure 4) is then inserted through the Access Cannula into the enucleated disc space. Contrast is injected into the inner chamber of the implant. This aids in visualization as well as provides real-time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a curable, polymer containing barium sulfate. The ...
Manufactured by:Zimmer Biomet based inWarsaw, INDIANA (USA)
The Echo Femoral Hip System offers a modern metaphyseal loading fit and fill design to address increasing hospital demands for both primary and fracture applications. Four stem options with various fixation modes address the distinct needs of each patient, while the customizable instrumentation platform provides enhanced surgical workflow. Zimmer Biomet addresses the unpredictability of fracture ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The SST PerQdisc Nucleus Replacement System is intended to replace the nucleus pulposus of the intervertebral disc in the L1-S1 spinal region in patients with single-level discogenic pain. The patient may have single or multi-level degenerative disc changes but the discogenic pain must be limited to a single level. The patient must be skeletally mature, at least 21 years of age, presenting with ...
Manufactured by:Think Surgical Inc. based inFremont, CALIFORNIA (USA)
The TSolution One Total Knee Application aids surgeons in executing their preoperative plans with precise control, performing hands-free milling for bone preparation. Other surgical systems merely guide the ...
Manufactured by:Kinamed Incorporated based inCamarillo, CALIFORNIA (USA)
Designed specifically for the small but challenging group of patients with isolated, end-stage patello-femoral disease, the KineMatch PFR (Patello-Femoral Replacement) offers a uniquely effective and conservative resurfacing solution. Because the device is precisely pre-fitted to the patient’s anatomy using CT (Computed Tomography) data, no resection of femoral bone is ...