- Home
- Equipment
- north america
- in vitro detection
Refine by
In Vitro Detection Equipment Supplied In North America
22 equipment items found
Manufactured by:Fujirebio based inMalvern, PENNSYLVANIA (USA)
A monoclonal globulin is used for the direct fluorescent antibody procedure in the in vitro detection of rabies in the brains and submaxillary glands of animals. This assay from Fujirebio Diagnostics detects all rabies and lyssaviruses tested to date including: street and fixed strains, Duvenhage, Lagos Bat, and Mokola. CE marked. ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
Maxim's FDA Approved HIV-1 Urine EIA test is an enzyme immunoassay for the in-vitro detection of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) in urine. The test is intended for use in professional laboratory settings as an aid in clinical diagnosis of HIV infection. Before a determination of HIV-1 status can be made, specimens that are repeatedly ...
Manufactured by:Creative Diagnostics based inShirley, NEW YORK (USA)
This product is suitable for in vitro quantiitative detection of Canine serum, plasma or cell culture supernatant and organizations in the natural and recombinant TPO concentration. Detection of other special sample please contact our technical support. The kit is for research use only. ...
Manufactured by:Scimedx Corporation based inDover, NEW JERSEY (USA)
Indirect Immunofluorescence Assay is a qualitative or semi-quantitative assay for the in vitro diagnostic detection of Anti-nuclear Antibodies in human serum on human epithelial cells (HEp2). The test is intended as an aid in the diagnosis of SLE and connective tissue ...
by:BioLab Sciences Inc. based inScottsdale, ARIZONA (USA)
BioLab Sciences is distributing Assure COVID-19 test kits. For more information, please click on the buttons below to open up the ...
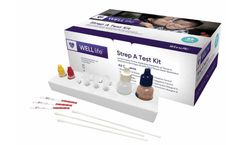
by:Wondfo USA Co Ltd. based inWillowbrook, ILLINOIS (USA)
The WELLlife Strep A Test Kit is a rapid chromatographic immunoassay intended for use by healthcare professionals as a qualitative in vitro diagnostic test for detection of group A streptococcal antigen from throat swab ...
Manufactured by:Azure Biotech Inc. based inHouston, TEXAS (USA)
This test has not been FDA cleared or approved. This test has been authorized by FDA under an EUA for use by authorized laboratories. It is a point of care test for fingerstick whole blood specimens only. The user should be trained in the procedure. Wear appropriate protective attire for your safety when handling patient ...
Manufactured by:BioGX, Inc. based inBirmingham, ALABAMA (USA)
The Xfree™ COVID-19 Direct RT-PCR test is offered lyophilized in a single vial, in the trusted BioGX Sample-Ready™ “Just Add Water” format where no other reagent is required. The test is available for the most ubiquitous real-time PCR platforms already available in thousands of laboratories. The fast and simple workflow is designed to help laboratories to increase their ...
Manufactured by:LG Chem, Ltd. based inSeoul, SOUTH KOREA
A reagent for in vitro diagnostic devices that detect semi-quantitatively allergen-specific IgE antibodies by immunoblotting in human serum or plasma treated with heparin, citrate, or CPDA-1 as an anticoagulant. (Results can be read by AdvanSure™ AlloView 1.0 only) ...
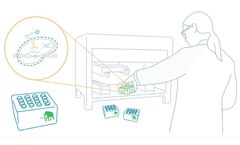
Manufactured by:Mammoth Biosciences, Inc. based inBrisbane, CALIFORNIA (USA)
A CRISPR-based detection solution for SARS-CoV-2. Mammoth Biosciences has developed a turnkey workstation to run a CRISPR-based SARS-CoV-2 molecular assay based on automated liquid handling equipment. This high throughput system can perform thousands of tests per day with minimal user interaction. CLIA high complexity laboratories will be able to significantly increase capacity with minimal FTE ...
Distributed by:UCP Biosciences Inc. based inSan Jose, CALIFORNIA (USA)
This kit is used for the in vitro quantitative detection of Cardiac Troponin I (cTnI), Creatine Kinase MB (CK-MB), Myoglobin (MYO) concentration in human serum/plasma and whole blood samples. Cardiac troponin I: cardiac troponin I (cTnI) is a subunit of the cardiac troponin complex that regulates actin and myosin interactions by regulating the activity of Ca2+ on ...
Manufactured by:LG Chem, Ltd. based inSeoul, SOUTH KOREA
A reagent for in vitro diagnostic devices that detect semi-quantitatively allergen-specific IgE antibodies by immunoblotting in human serum or plasma treated with heparin, citrate, or CPDA-1 as an anticoagulant. (Results can be read by AdvanSure™ AlloView 1.0 only) ...
Manufactured by:Premier Biotech, Inc. based inMinneapolis, MINNESOTA (USA)
The Premier Biotech COVID-19 IgG/IgM Rapid Test Cassette is a lateral flow immunochromatographic assay for the detection of SARS-CoV-2 antibodies in venous whole blood, serum or plasma. The COVID-19 IgG/IgM Rapid Test Cassette is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. COVID-19 (Coronavirus ...
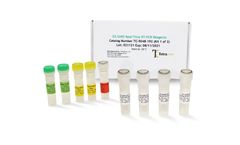
Manufactured by:Tetracore, Inc based inRockville, MARYLAND (USA)
EZ™-SARS-CoV-2 Real-Time RT-PCR is a real-time RT-PCR test intended for the qualitative detection of RNA from the SARS-CoV-2 in human nasal swab specimens from individuals suspected of COVID-19 by their healthcare provider. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity tests, ...
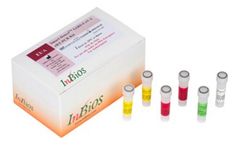
Manufactured by:InBios International, Inc based inSeattle, WASHINGTON (USA)
The SCoV-2 Detect™ IgG Rapid Test is an in vitro lateral flow chromatographic immunoassay intended for the qualitative detection of IgG antibodies to SARS-CoV-2 in human serum, plasma (sodium citrate, dipotassium EDTA and sodium heparin), venous whole blood (sodium citrate, dipotassium EDTA and sodium heparin), and fingerstick whole blood. ...
Manufactured by:Infitek Co., Ltd. based inJinan, CHINA
Dry Immunofluorescence Analyzer is used for in vitro quantitative detection of various indicators in human serum, plasma, whole blood, and urine.It is mainly used to detect the contents of PCT, hs-cTnI, NT-proBNP, H-FABP, CK-MB, MYO, D-Dimer, NGAL, etc., and the results are used for clinical auxiliary diagnosis. ...
Distributed by:UCP Biosciences Inc. based inSan Jose, CALIFORNIA (USA)
This kit is used for the in vitro quantitative detection of D-Dimer concentration in human plasma and whole blood samples. D-Dimer is a specific degradation product produced by fibrin monomer crosslinked with activation factor and hydrolyzed by plasmin. Its formation and elevation reflect the activation of coagulation and fibrinolytic system of the body and are ...
Manufactured by:InBios International, Inc based inSeattle, WASHINGTON (USA)
Smart DetectTM SARS-CoV-2 rRT-PCR Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) in human nasopharyngeal swab, anterior nasal swab and mid-turbinate nasal swab specimens from individuals suspected of COVID-19 by their healthcare provider. Clinical laboratories that are certified under ...
Manufactured by:Co-Diagnostics, Inc. based inSalt Lake City, UTAH (USA)
The Logix Smart™ SARS-CoV-2 DS (Direct Saliva) Test kit is an in vitro diagnostic test that uses our patented CoPrimer™ technology for the qualitative detection of two genes (gene RdRp and gene E) from the RNA of SARS-CoV-2 coronavirus (COVID-19), using a sample processing technique that eliminates the use of the nucleic acid extraction/purification ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
The 1st Point-of-Care (POC) Antibody Test for COVID-19 with Fingerstick Whole Blood Authorized by FDA!. Click the link to review FDA NEWS RELEASE on Sept 23, 2020 in details: "FDA Authorizes First Point-of-Care Antibody Test for COVID-19" about Assure COVID-19 IgG/IgM Rapid Test Device. This authorization means that Fingerstick Blood Samples can now be tested in POC settings like Doctor's ...