- Home
- Equipment
- north america
- infected medical
Refine by
Infected Medical Equipment Supplied In North America
41 equipment items found
Manufactured by:Scion BioMedical based inMiami, QUEBEC (CANADA)
Clo-SurPLUS P.A.D. offers hospitals and clinicians all the benefits of the Clo-Sur P.A.D. with the additional advantage as an Antimicrobial Barrier to protect against organisms that contribute to hospital-acquired infections. Clo-SurPLUS P.A.D. is the only non-invasive, topical hemostasic device currently cleared as an Antimicrobial ...
Manufactured by:Gentherm Medical based inNorthville, MICHIGAN (USA)
Gentherm’s Maxi-Therm® Lite hyper-hypothermia blankets have a strong bonded pattern in the blanket resulting in a long lasting seal during usage. The white material provides a comfortable, clean feel, and in today’s hospitals where hospital acquired infections are an important issue, having a white blanket helps notify the caregiver when the blanket is soiled and needs to be ...
Manufactured by:GCX Corporation based inPetaluma, CALIFORNIA (USA)
For enhanced cleanliness, minimal wear and damage, and easy servicing, the cable cover organizes and protects up to eight cables, and is easy to ...
Manufactured by:priMED Medical Products, Inc. based inEdmonton, ALBERTA (CANADA)
priMED’s success in Canadian hospitals has us working daily with clinicians. Throughout our collaborations we’ve discovered reliable and comfortable, single-use anesthesia masks are needed to reduce the risk of Hospital Acquired Infections (HAI’s). To meet this need, priMED introduced the PRIMAFORM and PRIMAFORM Grip—two types of anesthesia masks engineered to perform ...
Manufactured by:Ahlstrom based inEspoo, FINLAND
Ahlstrom Reliance, our single-use sterile barrier systems for wrap, overwrap and tray ...
Manufactured by:CleanSlate UV based inBuffalo, NEW YORK (USA)
Culture change powered by technology is the key to delivering safe, high-quality patient experience. CleanHands is a cloud-based sensor network that aids facilities in the battle against healthcare-acquired infections (HAIs) by providing complete transparency into hand hygiene ...
Manufactured by:Lumen Bioscience, Inc. based inSeattle, WASHINGTON (USA)
Traditionally considered a hospital-acquired infection, C. difficile is increasingly arising in the broader community. For this reason, the shelf-stable, orally delivered format of Lumen’s product is an important addition to the tool kit for treating and preventing this disease. Long-term consequences can include chronic diarrhea, severe intestinal inflammation, surgical resection, and ...
Manufactured by:VascuTech Medical, LLC based inOaks, PENNSYLVANIA (USA)
Dialysis presents a unique set of vascular access challenges from clotting to infection. VascuTech Medical is a leading contract manufacturer of clinical solutions for acute, chronic and peritoneal dialysis catheters with improved flow rates, reduced risk of clotting and increased patient ...
Manufactured by:Practivet, Inc. based inTempe, ARIZONA (USA)
The first and only FDA-cleared device shown to significantly reduce all types of reflux into a catheter and feature ICU Medical's proven infection control ...
Manufactured by:Sage Products LLC based inCary, ILLINOIS (USA)
Standards for proper meatal cleansing are not well-defined, which can lead to process variation. In addition to being inconsistent, traditional incontinence cleanup methods increase the risk of cross contamination from basins – a proven CAUTI risk factor – and waterborne hospital-acquired infections. Clean hands, fresh gloves, and a dedicated package of M-Care Meatal Cleansing Cloths ...
Manufactured by:Ready-Heat based inHoboken, NEW JERSEY (USA)
Cost effective way to keep every patient warm by preventing anesthesia induced hypothermia, increasing patient comfort for higher quality of care and an effective solution for infection control. Medical grade non-woven material, engineered thermal pads and proprietary high frequency welding constitute this original and most tested, fully-disposable patient ...
Manufactured by:Vedanta Biosciences, Inc based inCambridge, MASSACHUSETTS (USA)
VE707 is a preclinical discovery program for the prevention of infection and colonization recurrence of several multi-drug resistant organisms (MDROs) including carbapenem-resistant Enterobacteriaceae (CRE), extended-spectrum beta lactamase producers (ESBL), and vancomycin-resistant Enterococci (VRE), which are some of the most common hospital-acquired ...
Manufactured by:LifeAire Systems, LLC based inAllentown, PENNSYLVANIA (USA)
The Aire~HCX series in-duct modules offer the most comprehensive methodology to kill biologicals that can cause hospital acquired infections (HAIs) or subclinical infections. The systems have been genomically and mathematically modeled, and tested towards kill of the anthrax spore and the delivery of a 9-log reduction of biological ...
Manufactured by:SwipeSense, Inc. based inChicago, ILLINOIS (USA)
Culture change powered by technology is the key to delivering safe, high-quality health care. SwipeSense is a cloud-based sensor network that aids caregivers in the battle against hospital-acquired infections (HAIs) by providing complete transparency into hand hygiene data for each department, unit and individual. Our customer success team partners with hospitals to drive lasting ...
Manufactured by:Ahlstrom based inEspoo, FINLAND
Our soft SMS fabric just got even better by adding an exclusive dynamic cooling treatment. Introducing Ahlstrom ...
Manufactured by:Galbino Technology Inc. based inMarkham, ONTARIO (CANADA)
The Galbino ZW2211A series filter container has been specifically engineered for optimal performance in hospital sterile supply environments. It is constructed from high-quality materials that facilitate efficient heat conduction and exhibit excellent corrosion resistance while maintaining a lightweight profile. The series offers the convenience of either disposable or reusable filters to enhance ...
Manufactured by:Baxter International Inc. based inDeerfield, ILLINOIS (USA)
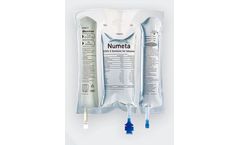
NUMETA G13E 300 mL is the only triple-chamber, ready-to-use parenteral (intravenous) nutrition (PN) product available to treat preterm infants (less than 37 weeks gestation age) who are at high risk for infection and malnutrition5 in the early hours and days of their ...
Manufactured by:Ahlstrom based inEspoo, FINLAND
Ahlstrom offers an extensive range of surgical drapes that offer protection and ...
Manufactured by:Newmatic Medical based inCaledonia, MICHIGAN (USA)
Medical Face Mask. 17.5 x 9.5cm - 3 ply Non-Woven, with ear loop, blue color. Complies with ASTM F2100 Level 2 requirements. Testing specifications are available upon request. ASTM F 2101-14 BFE > 98% at 3 microns. ASTM F2299 PFE = 98% at 0.1 microns. Delta P, mm ...
Manufactured by:Xodus Medical Inc. based inNew Kensington, PENNSYLVANIA (USA)
Safety Maximized: Increase patient safety with Pink Lat Packs; featuring breathable, moisture-wicking materials that immerse and envelope areas prone to pressure ...