- Home
- Equipment
- north america
- inflammation leading
Show results for
Refine by
Inflammation Leading Equipment Supplied In North America
22 equipment items found
Manufactured by:Altimmune Inc. based inGaithersburg, MARYLAND (USA)
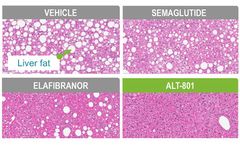
A peptide-based dual GLP-1/glucagon receptor agonist designed to treat the metabolic dysfunction that causes non-alcoholic steatohepatitis (NASH). NASH, the most severe form of non-alcoholic fatty liver disease (NAFLD), involves multiple metabolic pathways leading to the abnormal accumulation of liver fat, toxic lipid metabolites, and inflammation, ...
Manufactured by:Medinol based inTel Aviv, ISRAEL
WiZeCell, our proprietary stent design is paradigm shift in cardiovascular stenting. The design’s sixth generation, the EluNIR drug eluting stent, relieves physicians from compromising between vessel conformability, scaffolding integrity, uniform drug distribution and radial support by offering an integrated design maximizing all benefits ...
Manufactured by:Shape Memory Medical Inc. based inSanta Clara, CALIFORNIA (USA)
The biocompatible and non-inflammatory polymer promotes rapid conversion to organized thrombus, followed by collagen deposition without chronic active inflammation, which leads to a stable occlusion. ...
Manufactured by:Shape Memory Medical Inc. based inSanta Clara, CALIFORNIA (USA)
The biocompatible and non-inflammatory polymer promotes rapid conversion to organized thrombus, followed by collagen deposition without chronic active inflammation, which leads to a stable occlusion.‡ The anchor coil offers stability in higher-flow locations and IMPEDE may be used in combination with IMPEDE-FX to minimize artifact in a single ...
Manufactured by:Shape Memory Medical Inc. based inSanta Clara, CALIFORNIA (USA)
The biocompatible and non-inflammatory polymer promotes the rapid conversion to organized thrombus, followed by collagen deposition without chronic active inflammation, which leads to a stable occlusion. ...
Manufactured by:BiomX based inNess Ziona, ISRAEL
BX003 is an orally administered phage cocktail targeting a bacterial target present in the gut of IBD and PSC patients and thought to be associated with the onset or exacerbation of these diseases. Although different organs are affected in IBD and PSC (gut in IBD and liver in PSC) the two diseases are thought to be related since approximately 70% of PSC patients also suffer from IBD. Results from ...
Manufactured by:KORU Medical Systems based inMahwah, NEW JERSEY (USA)
This is caused by an autoimmune process in which the body’s own immune system doesn’t recognize its nerve fibers and attacks them. This leads to inflammation of nerve roots and peripheral nerves, as well as the deterioration of the fatty protective covering (myelin sheath) over the nerves, affecting how fast the nerve signals are transmitted and ...
Manufactured by:PAVmed Inc. based inNew York, NEW YORK (USA)
CarpX* is a minimally invasive device designed to treat carpal tunnel syndrome, allowing the physician to relieve the compression of the median nerve without a traditional surgical incision. CarpX incorporates a balloon catheter with bipolar radio-frequency cutting electrodes. The device is advanced over a wire and positioned in the carpal tunnel under ultrasonic guidance. When activated, it ...
Manufactured by:BioMarin based inSan Rafael, CALIFORNIA (USA)
Kuvan® (sapropterin dihydrochloride) Tablets and Powder for Oral Solution is the first FDA-approved medication for phenylketonuria (PKU). Kuvan is a form of BH4, the cofactor of the PAH enzyme, which helps the enzyme break down Phe. Kuvan is to be used in conjunction with a Phe-restricted ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc is designed for use using a lateral traspsoas or retroperitoneal (i.e., anterolateral) surgical approach to access disc space. Access through the annulus can be achieved using the PerQdisc™ Disc Access System (Figure 3). Once annular access is achieved, a nuclectomy (removal of nucleus material) is performed through the disc access using various tools to remove most of the ...
Manufactured by:Quidel Corporation based inSan Diego, CALIFORNIA (USA)
Chlamydia is a genus of bacterial pathogens that includes Chlamydia trachomatis and Chlamydia pneumonia. C. pneumonia is a common cause of atypical pneumonia and C. trachomatis causes the most common sexually transmitted ...
by:Aegle Therapeutics based inWoburn, MASSACHUSETTS (USA)
Epidermolysis bullosa (“EB”) is a group of rare genetic disorders that manifests as blistering or erosion of the skin in response to little or no apparent trauma. There are many genetic and symptomatic variations of EB. EB is always painful, often pervasive and debilitating, and is in some cases lethal before the age of 30. EB affects both genders and every racial and ethnic ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The PerQdisc Implant Delivery Device (Figure 4) is then inserted through the Access Cannula into the enucleated disc space. Contrast is injected into the inner chamber of the implant. This aids in visualization as well as provides real-time monitoring of fill pressure. The outer chamber of the implant is then filled under pressure control with a curable, polymer containing barium sulfate. The ...
by:Cartesian Therapeutics based inGaithersburg, MASSACHUSETTS (USA)
The current standard of care for autoimmune diseases is broad immunosuppression, which is associated with side effects and leaves patients vulnerable to serious infection and maligancies. Our approach to autoimmune disease is designed to restore natural self-tolerance by administering ImmTOR with nanoparticles-encapsulated self-antigens and avoid the need for chronic and systemic immune ...
Manufactured by:Bio-Tissue, Inc. based inMiami, FLORIDA (USA)
PROKERA biologic corneal bandage devices are used by eye doctors around the world to heal and treat ocular conditions such as keratitis, moderate to severe dry eye disease, recurrent corneal erosions, filamentary keratitis, persistent epithelial defects, neurotrophic corneas, herpetic ulcers, and many other ocular surface diseases such as chemical burns and Stevens-Johnson syndrome. The PROKERA ...
Manufactured by:Spinal Stabilization Technologies, LLC based inKilkenny City, IRELAND
The SST PerQdisc Nucleus Replacement System is intended to replace the nucleus pulposus of the intervertebral disc in the L1-S1 spinal region in patients with single-level discogenic pain. The patient may have single or multi-level degenerative disc changes but the discogenic pain must be limited to a single level. The patient must be skeletally mature, at least 21 years of age, presenting with ...
Manufactured by:Bio-Tissue, Inc. based inMiami, FLORIDA (USA)
PROKERA biologic corneal bandage devices are used by eye doctors around the world to heal and treat ocular conditions such as keratitis, moderate to severe dry eye disease, recurrent corneal erosions, filamentary keratitis, persistent epithelial defects, neurotrophic corneas, herpetic ulcers, and many other ocular surface diseases such as chemical burns and Stevens-Johnson syndrome. The PROKERA ...
Manufactured by:Microbion Corporation based inBozeman, MONTANA (USA)
Cystic Fibrosis (CF) lung disease, which is estimated to affect more than 30,000 in the US and 70,000 worldwide1, is characterized by chronic bacterial infection and severe inflammation that leads to progressive deterioration in lung function. Chronic pulmonary infections in CF patients, a significant portion which involves Pseudomonas aeruginosa, are ...
by:Alucent Biomedical Inc. based inSalt Lake City,, UTAH (USA)
PAD is a common circulatory problem in which narrowed arteries reduce blood flow to the extremities – generally affecting one or both legs. It is caused when the inner lining of arteries located outside of the heart get damaged by factors such as smoking, obesity, high blood pressure, elevated cholesterol, elevated triglyceride levels, and/or diabetes. Once vessels are damaged, plaque can ...
Manufactured by:Sepset Biosciences Inc. based inVictoria, BRITISH COLUMBIA (CANADA)
Sepset is developing a blood test for hospitals to identify septic patients in the emergency room (ER) and predict those who will have high risk for organ ...