- Home
- Equipment
- north america
- minimally invasive single site surgery
Refine by
Minimally Invasive Single Site Surgery Equipment Supplied In North America
104 equipment items found
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
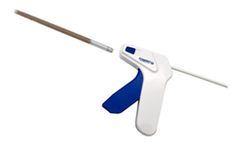
The BioSphere® MIS Putty graft delivery system is precision engineered to extrude BioSphere® Putty through a cannula designed for minimally invasive surgery ...
Manufactured by:Synergy Biomedical, LLC based inWayne, PENNSYLVANIA (USA)
The BioSphere MIS II Putty graft delivery system is precision engineered to extrude BioSphere® Putty through a cannula designed for minimally invasive surgery ...
Manufactured by:DeRoyal Industries, Inc. based inPowell, INDIANA (USA)
Easy to use telescopic fold with cuff. Clear, elasticized fenestration for secure fit around endoscope. Optional adhesive tape strip and rubber bands to further secure drape closure. Stretchable neck material ensures tight fit. Sterile, Not made with natural rubber ...
Manufactured by:Aspen Surgical based inCaledonia, MICHIGAN (USA)
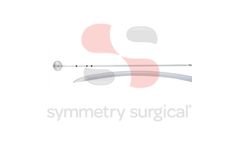
NLA Symmetry Reddick Cholangiogram Catheter, Scoop Tip, 4 Fr, 50 cm, Single ...
Manufactured by:Life Spine, Inc. based inHuntley, ILLINOIS (USA)
The CALYPSO Midline Retractor System is a low-profile, minimally invasive system designed to offer optimal access and visibility to the surgical site. Minimally invasive midline approach reduces OR time and complications. Snap on quick release blades enhance ease of use while attaching/detaching to the retractor. Anatomically shaped blade teeth conform to the facet joint for improved retraction. ...
Manufactured by:Amplify Surgical based inIrvine, CALIFORNIA (USA)
The dualX systems transform the fusion environment from insertion to spinal restoration by delivery a powerful dual-expanding implant through a minimally-invasive surgical corridor designed to reduce anatomical trauma and nerve retraction. ...
Manufactured by:Applied Medical Resources Corporation based inRancho Santa Margarita, CALIFORNIA (USA)
The Alexis Contained Extraction System (Alexis CES) creates a contained environment for tissue extraction during minimally invasive surgery. The Alexis CES features a specimen containment bag and a guard to protect the bag from sharp instrumentation. It is available in several kit configurations to support multi-port, reduced port and single incision techniques. ...
Manufactured by:Spinologics Inc based inMontréal, QUEBEC (CANADA)
The MIThora is a reusable trocar designed to establish a port of entry for thoracoscopic instruments used during minimally invasive surgery by anterior approach. The MIThora has a trocar cannula and a trocar obturator equipped with a ball handle. The trocar cannula has an internal diameter of 17mm and is 127mm long. The MIThora can be delivered in a polybag, or within a sterilization ...
Manufactured by:Amplify Surgical based inIrvine, CALIFORNIA (USA)
The dualX systems transform the fusion environment from insertion to spinal restoration by delivery a powerful dual-expanding implant through a minimally-invasive surgical corridor designed to reduce anatomical trauma and nerve retraction. ...
Manufactured by:Amplify Surgical based inIrvine, CALIFORNIA (USA)
The dualX systems transform the fusion environment from insertion to spinal restoration by delivery a powerful dual-expanding implant through a minimally-invasive surgical corridor designed to reduce anatomical trauma and nerve retraction. ...
Manufactured by:Clinicon Corporation based inOceanside, CALIFORNIA (USA)
The PureBeam delivery system is the preferred tool of minimally invasive surgery specialists to treat soft tissue disorders in the field of Neuro-, Spine-, and ENT ...
Manufactured by:Instrument Technology, Inc. (ITI) based inWestfield, MASSACHUSETTS (USA)
Key Features: Rigid or semi-rigid, articulating. Applications: Minimally invasive sound imaging and ...
Manufactured by:Novuson based inBothell, WASHINGTON (USA)
Why 3 mm? It’s simple, the smaller the diameter of the surgical instrument, the smaller the incision required, which should result in a quicker healing time and virtually no scar. In minimally invasive surgery, smaller is better! Our 3mm Ultrastat LS will be capable of sealing and dividing vessels up to ...
Manufactured by:Tegra Medical based inFranklin, MASSACHUSETTS (USA)
Highlights: Swaging, bending, flaring, and coining. Sheet metal stamping. Augmented by nitinol shape ...
by:ni2o, Inc. based inProvidence, RHODE ISLAND (USA)
Our Kinetic Intelligent Wireless Implant (KIWI) is a small, self-contained, wirelessly connected, wirelessly charged, AI-driven replacement for current deep brain stimulation (DBS) solutions. KIWI will record brain activity and deliver electrical and optical signals to target areas, with algorithms that will determine the therapeutic response and be continually improved by machine learning. It is ...
Manufactured by:Origami Surgical LLC based inMadison, NEW JERSEY (USA)
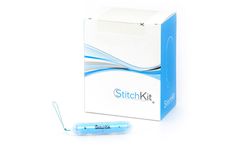
Indication for Use: StitchKit® Suture Delivery Canister facilitates minimally invasive robotic surgery by transporting suture to the operative field and removing used needles after suturing. The suture contained within the StitchKit® device is intended for soft tissue approximation where use of the specific absorbable or non-absorbable sutures contained within it is ...
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
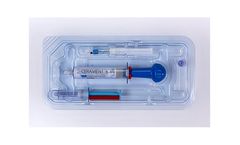
CERAMENT BONE VOID FILLER is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent iohexol. The unique ratio of hydroxyapatite and calcium sulfate is designed to enable CERAMENT to resorb at the same rate that bone forms. Calcium sulfate acts as a resorbable carrier for hydroxyapatite, and hydroxyapatite ...
Manufactured by:Origami Surgical LLC based inMadison, NEW JERSEY (USA)
Indication for Use: StitchKit® Suture Delivery Canister facilitates minimally invasive robotic surgery by transporting suture to the operative field and removing used needles after suturing. The suture contained within the StitchKit® device is intended for soft tissue approximation where use of the specific absorbable or non-absorbable sutures contained within it is ...
Manufactured by:NUVO based inErie, PENNSYLVANIA (USA)
The Nuvo Vu LED Surgical Light is brighter, whiter, cooler and more capable in the operating room than any previous technology. So you get the most accurate colors with unparalleled shadow control — and virtually no heat emission. All this for a value that’s hard to match. Cross Focus Technology enables the LED’s to move independently of each other while adjusting the pattern ...
Manufactured by:MedSpark, LLC based inSan Luis Obispo, CALIFORNIA (USA)
During Total Knee Arthroplasty (replacement) or TKA, a patellar component (commonly composed of UHMW polyethylene) is typically located by the surgeon on the posterior aspect of the patient's existing patella. The patellar surface must be prepared by the surgeon to accept the patellar component and a patella clamp instrument is typically utilized by surgeons during this ...