- Home
- Equipment
- north america
- nasal spray
Refine by
Nasal Spray Equipment Supplied In North America
13 equipment items found
Manufactured by:Emergent BioSolutions Inc. based inGaithersburg, MARYLAND (USA)
You and your family members or caregivers should read this Patient Information leaflet before an opioid emergency happens. This information does not take the place of talking with yourhealthcare provider about your medical condition or your ...
Manufactured by:Kerecis based inArlington, VIRGINIA (USA)
Viruxal – Additional protection against viral infections. Viruxal is an additional protection against viral infections that is available as an oral and nasal spray. Viruxal is a medical device that creates a temporary protective barrier when sprayed into the nose and mouth. It helps reduce viral load by preventing illness-causing airborne ...
Manufactured by:Zydus Pharmaceuticals (USA) Inc. based inPennington, NEW JERSEY (USA)
Strength: 0.01%. Description: Bottle Pump. Imprint Code: N/A. Rating: AB. Brand Equivalent: DDAVP. Therapeutic Category: Pituitary. Product Status: Active. ...
Manufactured by:Auris Medical AG based inBasel, SWITZERLAND
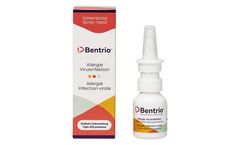
Bentrio™ is a drug-free nasal spray for personal protection against airborne viruses and allergens. Upon application into the nose, Bentrio™ forms a protective gel layer on the nasal mucosa. This thin film is designed to prevent the contact of viruses or allergens with cells; in addition, the composition serves to bind such particles ...
Manufactured by:MuseChem based inPaoli, PENNSYLVANIA (USA)
Cetylpyridinium Chloride(CAT: A000659) is an antibacterial agent that kills bacteria and other microorganisms, preventing plaque and reducing gingivitis. It is a component of certain mouthwashes, toothpaste, pastilles, throat sprays, nasal sprays, and breathing sprays. This product is used for organic synthesis, pharmaceutical ...
Manufactured by:Proveris Scientific Corporation based inHudson, MASSACHUSETTS (USA)
SprayVIEW Measurement Systems by Proveris are industry-standard tools designed to evaluate the spray patterns and plume geometry of orally inhaled and nasal drug products (OINDPs). These systems are crucial for both development and quality control phases, ensuring compliance with FDA guidelines and providing bioequivalence assessments. The system's adaptability ...
Manufactured by:Proveris Scientific Corporation based inHudson, MASSACHUSETTS (USA)
Vereo Automated Actuators are designed for precise control in the testing and development of nasal and oral spray devices. These actuators support various configurations, including vertically, side, and dual side-actuated devices, reducing errors from manual testing and enhancing data quality. The electromechanical design eliminates the need for compressed air or ...
Manufactured by:Auris Medical AG based inBasel, SWITZERLAND
Development of Bentrio™ (AM-301) has been completed. The product has been introduced to the market, starting in selected countries of the European ...
Manufactured by:SEQENS Group based inÉcully, FRANCE
Fluticasone is a steroid with glucocorticoid receptor activity that is used to manage the symptoms of asthma; allergic rhinitis, and atopic ...
Manufactured by:Intersect ENT, Inc. based inMenlo Park, CALIFORNIA (USA)
SINUVA is the first and only FDA-approved stent for the treatment of nasal polyps in adult patients 18 years or older who have had ethmoid sinus surgery Are you still living with nasal polyps? Consider an alternative to another sinus surgery — SINUVA is a nonsurgical treatment option that can be placed in office. SINUVA delivers anti-inflammatory medicine directly to the nasal polyps, ...
Manufactured by:Lyra Therapeutics, Inc. based inWatertown, MASSACHUSETTS (USA)
Lyra’s lead product candidate, LYR-210, to treat chronic rhinosinusitis (CRS), an inflammatory disease of the paranasal sinuses. LYR-210 is an anti-inflammatory implantable drug matrix based upon Lyra’s XTreo™ platform designed to elute mometasone furoate consistently and locally to the inflamed mucosal tissue of surgically-naïve CRS patients for up to six months from a ...
Manufactured by:Altimmune Inc. based inGaithersburg, MARYLAND (USA)
Identical vector technology used for AdCOVID (COVID-19), NasoVAX (seasonal influenza) and NasoShield (anthrax) ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Fluticasone furoate is a topical, intranasal, enhanced-affinity synthetic trifluorinated corticosteroid with a Kd of 0.3 nM. Fluticasone furoate has potent anti-inflamatory and anti-asthmatic activity, and low systemic exposure. Fluticasone furoate has the potential for allergic rhinitis ...