- Home
- Equipment
- north america
- nucleic acid extraction
Show results for
Refine by
Nucleic Acid Extraction Equipment Supplied In North America
10 equipment items found
Manufactured by:BioGX, Inc. based inBirmingham, ALABAMA (USA)
The test is offered in BioGX’s trusted, easy to use Sample-Ready™ format where all required reagents for real-time PCR are lyophilized in a single tube. BioGX provided tube snaps into a test-specific position on the BD MAX™ total nucleic acid extraction cartridge enabling Sample-to-Answer molecular testing. BioGX kits can be ...
Manufactured by:Co-Diagnostics, Inc. based inSalt Lake City, UTAH (USA)
The Logix Smart™ SARS-CoV-2 DS (Direct Saliva) Test kit is an in vitro diagnostic test that uses our patented CoPrimer™ technology for the qualitative detection of two genes (gene RdRp and gene E) from the RNA of SARS-CoV-2 coronavirus (COVID-19), using a sample processing technique that eliminates the use of the nucleic acid ...
Manufactured by:Ceres Nanosciences, Inc. based inManassas, VIRGINIA (USA)
Compatible with PCR, sequencing, or immunoassay analysis methods. Enables rapid and simple automated or manual methods for preparing wastewater samples for nucleic acid extraction, without filtration, centrifugation, or bead-beating. Successfully utilized in high-complexity and point-of-care RT-PCR assays with FDA Emergency Use Authorization for ...
Manufactured by:Ceres Nanosciences, Inc. based inManassas, VIRGINIA (USA)
Compatible with PCR, sequencing, or immunoassay analysis methods. Enables rapid and simple automated or manual methods for preparing wastewater samples for nucleic acid extraction, without filtration, centrifugation, or bead-beating. Successfully utilized in high-complexity and point-of-care RT-PCR assays with FDA Emergency Use Authorization for ...
Manufactured by:Copan Diagnostics Inc. based inMurrieta, CALIFORNIA (USA)
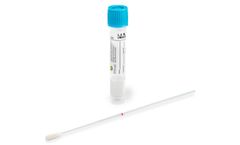
MSwab® is a collection, transport and preservation system designed for rapid direct nucleic acid heat extraction for real-time PCR and isothermal amplification for molecular assays. MSwab® has the advantage of enabling reflex culture for traditional culture of gram-positive aerobic and facultative anaerobic ...
by:Thermo Fisher Scientific, LIMS & Laboratory Software based inPhiladelphia, PENNSYLVANIA (USA)
Specimen to report in a single day with a hands-off, automated workflow. The Ion Torrent Genexus System is the first turnkey next-generation sequencing (NGS) solution that automates the specimen-to-report workflow and delivers results in a single day with just two user touchpoints.* This highly flexible system lets you process samples—even just one—cost-effectively as they come in. ...
Manufactured by:Biomed Diagnostics, Inc. based inWhite City, OREGON (USA)
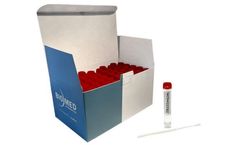
Biomed Diagnostics' VTM-C19 Transit Tube is intended for on-site collection and transport to the testing laboratory of human clinical specimens containing SARS-CoV-2 — the virus that causes COVID-19 disease in humans and/or (USA only) influenza A virus. Intended to be inoculated with specimens collected with a nasopharyngeal (NP) or oropharyngeal (OP) synthetic fiber swab (not provided), ...
Manufactured by:ELITechGroup based inPuteaux, FRANCE
ELITe InGenius is an easy-to-use sample-to-result solution dedicated to Molecular Diagnostics which integrates extraction, amplification and result interpretation with an unprecedented flexibility and assay menu ...
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
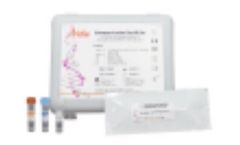
The Aridia Chikungunya Real-Time PCR Test is based on the real time amplification and detection of chikungunya virus (CHIKV) RNA in a one-step ...
by:Diasorin based inSALUGGIA, ITALY
The VERIGENE System enables clinicians to rapidly identify the pathogens responsible for some of the most complex, costly, and deadly infectious ...