- Home
- Equipment
- north america
- parenteral
Show results for
Refine by
Parenteral Equipment Supplied In North America
34 equipment items found
Manufactured by:ICU Medical, Inc. based inSan Clemente, CALIFORNIA (USA)
ICU Medical is committed to helping you deliver more comprehensive patient-care therapies. Our trusted portfolio of parenteral nutrition products puts a variety of options at your fingertips, delivering an extensive source of nutrients for patients who cannot consume a normal diet. Working closely with industry leaders, we are continuously improving and investing in our ...
Manufactured by:ZebraSci Inc. based inTemecula, CALIFORNIA (USA)
Residual Seal Force (RSF) is a non-destructive method to evaluate seal tightness in the stopper/seal combination of parenteral vials. Parenteral vial systems consist of a vial, a stopper and an overseal. Capping parameters are a critical aspect during drug product manufacturing. During the sealing process, improper capping can generate cosmetic defects or worse ...
Manufactured by:Sealed Air Corporation based inCharlotte, NORTH CAROLINA (USA)
NEXCEL® brand primary medical films are ideal for large volume parenterals and multi-chamber ...
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Pentagastrin is a synthetic pentapeptide that has effects like gastrin. When given parenterally it stimulates the secretion of gastric acid, pepsin and intrinsic factor.http://www.creative-peptides.com/product/pentagastrin-item-10-101-95-84.html ...
Manufactured by:Facet Analytical Services & Technology LLC based inPunta Gorda, FLORIDA (USA)
Small Vial filling 2 mL – 20 mL, threaded or crimp cap. Serum Vial or Bottle, USP Type I, II, or III. Clear or Amber glass. Large parenteral infusion bottles, 30 mL – 500 mL. Pharmaceutical stoppering (2-leg lyo, igloo, plug, serum). 13 mm, 20 mm, and 28 mm crimp seal with color coding options. 3-piece tear-off and tamper evident ...
Manufactured by:ApiJect Systems, Corp. based inStamford, CONNECTICUT (USA)
The ApiJect Platform brings together prefilled, single-dose parenterals with scalable, efficient manufacturing to solve drug delivery challenges for sterile liquid pharmaceuticals in all markets. ...
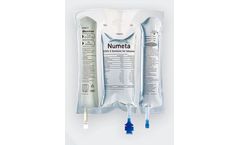
Manufactured by:Baxter International Inc. based inDeerfield, ILLINOIS (USA)
NUMETA G13E 300 mL is the only triple-chamber, ready-to-use parenteral (intravenous) nutrition (PN) product available to treat preterm infants (less than 37 weeks gestation age) who are at high risk for infection and malnutrition5 in the early hours and days of their ...
Manufactured by:Keystone Folding Box Co. based inNewark, NEW JERSEY (USA)
Healthcare professionals and caregivers, including staff in hospitals, assisted living facilities and at-home care settings, need vial boxes with simple instructions and easy storage that protect products and support ease of parenteral drug ...
Manufactured by:SyncMedical LLC based inStoughton, MASSACHUSETTS (USA)
The Primo Port is a fully implantable vascular access device that meets the requirements of clinicians and patients by providing long-term repeated access to the vascular system. The Primo Port system can be used for infusion of medications, IV fluids, parenteral nutrition and blood products as well as for the aspiration of blood ...
Manufactured by:Keystone Folding Box Co. based inNewark, NEW JERSEY (USA)
Biologics, biosimilars, vaccines and other medications administered through pre-filled syringes present unique secondary packaging challenges. First and foremost, product protection is paramount, as many parenteral pharmaceuticals are packaged in glass or rigid plastic and can be prone to breakage or cracking. Secondary packaging solutions must have the right materials so that ...
Manufactured by:Stanford Chemicals Company based inLake Forest, CALIFORNIA (USA)
Injection Grade Sodium Hyaluronate is widely used for parenteral preparations including intra-articular and intra-ocular preparations, such as OVD, intra-articular injections, intradermal injections for aesthetic correction, anti-adhesive products, ...
Manufactured by:Raumedic AG based inDietzenbach, GERMANY
Containers made of EVA are ideal for high-quality primary packaging solutions such as infusion and feeding bags for enteral and parenteral nutrition (TPN, IV nutrition ...
Manufactured by:Emergent BioSolutions Inc. based inGaithersburg, MARYLAND (USA)
Cases of true hypoglycemia may go untreated if the hypoglycemic state is masked by falsely elevated glucose readings.Carefully review t he product information of the blood glucose testing system, including that of the test strips, to determine if the system is appropriate for use with maltose-containing parenteral products [see 5.3 Blood Glucose ...
Manufactured by:Applied Medical Technology, Inc. (AMT) based inBrecksville, OHIO (USA)
Reports show that 40 percent of nasogastric feeding tubes are dislodged, which may lead to the unnecessary surgical placement of a feeding device or conversion to parenteral nutrition support.* A nasal bridle is an effective and safe way to secure a patient’s nasal tube, retaining the nutrition flow to the ...
Manufactured by:Protara Therapeutics, Inc. based inNew York, NEW YORK (USA)
IV choline chloride is an investigational, intravenous (IV) phospholipid substrate replacement therapy initially in development for patients receiving parenteral nutrition (PN) who have intestinal failure-associated liver disease (IFALD). Choline is a known important substrate for phospholipids that are critical for healthy liver function. Because PN patients cannot ...
Manufactured by:Stanford Chemicals Company based inLake Forest, CALIFORNIA (USA)
For parenteral preparations or oral preparations not including intra-articular and intra-ocular preparations. It can be used in eye drops, contact lens solutions, topical preparations for the wound or burn healing, medical lubricants, ...
Manufactured by:Getinge AB based inGöteborg, SWEDEN
Clean, sterile, and dry stoppers and closures delivered to the filling machine without breaking containment. The Getinge Closure Processing System (CPS) provides an unbroken chain of sterility for efficient processing of all types of closures in conjunction with filling lines in pharmaceutical ...
Manufactured by:InHealth Technologies based inCarpinteria, CALIFORNIA (USA)
Blom-Singer Adjustable Bi-Flanged Fistula Prosthesis is made from medical grade silicone and is designed to reduce leakage of saliva, food and drink around a Hypopharyngeal Fistula. The prostheses come in three sizes, 25mm, 38mm, and 50mm, ensuring a proper ...
Manufactured by:Terumo Blood and Cell Technologies, Inc. based inLakewood, COLORADO (USA)
Reduce variability of contamination in your process by standardizing sterile tubing connections with a functionally closed system. ...
Manufactured by:ApiJect Systems, Corp. based inStamford, CONNECTICUT (USA)
The Prefilled ApiJect Injector* is a delivery system designed to bring together the benefits of prefilled syringes with the manufacturing efficiency of multi-dose ...