- Home
- Equipment
- north america
- patient isolation
Refine by
Patient Isolation Equipment Supplied In North America
22 equipment items found
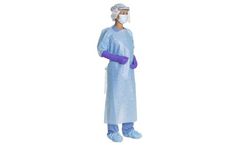
Manufactured by:SRI Healthcare based inAtlanta, GEORGIA (US) (USA)
Reusable Isolation Gowns ensures continuity of supply while protecting the environment and provides savings for the hospital. Isolation Gowns are a Non-Surgical medical device intended to protect the wearer from the transfer of microorganisms and body fluids in low or minimal risk patient isolation situations ...
Manufactured by:HDT Global based inSolon, OHIO (USA)
The HDT Medical Shelter System for patient isolation is a complete, rapidly-deployed, mobile shelter system that provides effective airborne infection control and isolation. Featuring our world-standard Base-X® shelters, the system includes an EMAT-3T Bio Filter / Environmental Control Unit that uses HEPA filtration and ultraviolet germicidal ...
Manufactured by:Covalon Technologies Ltd based inMississauga, ONTARIO (CANADA)
The chlorhexidine and silver contained in the adhesive provides continuous antimicrobial activity while the polyurethane barrier film acts as a protective patient covering to isolate a procedural site from microbial and other ...
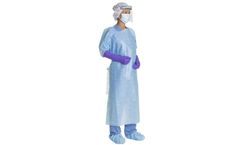
Manufactured by:Owens & Minor, Inc. based inMechanicsville, VIRGINIA (USA)
These non-sterile gowns feature an impervious plastic film or film laminate, poly-coated spunbond, or 3-layer SMS fabric with polyethylene coating. The garments combine effective fluid resistance with wearer comfort. These gowns are ideal for patient contact, fluid isolation, decontamination or general cleanup tasks. ...
Manufactured by:O & M Halyard, Inc. based inAlpharetta, GEORGIA (US) (USA)
These non-sterile gowns feature an impervious plastic film or film laminate, poly-coated spunbond, or 3-layer SMS fabric with polyethylene coating. The garments combine effective fluid resistance with wearer comfort. These gowns are ideal for patient contact, fluid isolation, decontamination or general cleanup tasks. ...
Manufactured by:Vivosonic Inc. based inToronto, ONTARIO (CANADA)
Portable wireless technology for clinical convenience. The VivoLink™ is the world’s first and only wireless platform for auditory electrophysiological and otoacoustic emission assessment. With no wires connecting the patient to the computer, there is greater freedom of movement for patients and convenience for clinicians. The VivoLink™ allows a baby to be held or strolled, and ...
Manufactured by:Cepheid based inSunnyvale, CALIFORNIA (USA)
Important healthcare benefits of rapid detection: Helps ensure the right therapy sooner for improved patient management. Enables a targeted antimicrobial therapy approach for better antimicrobial stewardship. Allows for immediate identification of patient infection for improved isolation and infection control ...
Manufactured by:EntroGen, Inc. based inWoodland Hills, CALIFORNIA (USA)
EntroGen RNA Fusion Gene Panel is a complete next generation sequencing (NGS) solution for detecting 307 clinically relevant fusions over 23 genes. ...
Manufactured by:Galaxy Diagnostics, Inc. based inResearch Triangle Park, NORTH CAROLINA (USA)
When Bartonella bacteria are present in the body, the immune system creates antibodies that help eliminate them. Test methods, such as immunofluorescence assays (IFA), determine the level of IgM and/or IgG antibodies circulating in a patient, which can be useful for diagnosis of bartonellosis and monitoring treatment response. Almost 20 Bartonella species have been implicated in human and animal ...
Manufactured by:ELITechGroup based inPuteaux, FRANCE
Aspergillosis is the most common cause of fungal infection among stem cell and solid organ transplant recipients, resulting in high mortality if untreated. Patients with weakened immune system and lung diseases are at higher risk of developing severe outcomes. Early detection and quantification of Aspergillus with molecular methods is crucial to isolate infected ...
Manufactured by:Orchestra BioMed, Inc. based inNew Hope, PENNSYLVANIA (USA)
BackBeat CNT™ is a patented bioelectronic treatment for hypertension that is designed to drive an immediate, substantial and persistent reduction in blood pressure while simultaneously modulating autonomic nervous system responses to maintain lowered blood pressure long-term. A patented, intelligent bioelectronic treatment for hypertension. BackBeat Cardiac Neuromodulation Therapy is ...
Manufactured by:Engenious Design based inPrairie Village, KANSAS (USA)
Our experience enables us to understand the unique challenges of this technology. Over their careers, our team has been part of designing 11 electrosurgical systems for various therapies ranging from vascular closure and nerve ablation to general surgery. This experience includes single-electrode, multi-electrode, monopolar and bipolar applications, ablation/cut/coag/gas plasma ...
Manufactured by:Lumos Diagnostics based inSarasota, FLORIDA (USA)
CoviDx™ SARS-CoV-2 Rapid Antigen Test is a lateral flow assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasal swabs and nasopharyngeal swabs from patients suspected of a Coronavirus-19 (COVID-19) infection by their healthcare provider within the first 5 days of ...
Manufactured by:Hemogenyx Pharmaceuticals plc based inLondon, UNITED KINGDOM
Hemogenyx Pharmaceuticals has constructed Chimeric Antigen Receptor (CAR) programmed T cells, termed HEMO-CAR-T, for the potential treatment of Acute Myeloid Leukemia (AML). HEMO-CAR-T is made using Hemogenyx’s proprietary humanised monoclonal antibody against a target on the surface of AML ...
Manufactured by:EntroGen, Inc. based inWoodland Hills, CALIFORNIA (USA)
BRCA Complete Expanded Panel is a complete next generation sequencing (NGS) solution for detecting clinically relevant BRCA1 and BRCA2 mutations along with extended coverage of CHEK2, PALB2, RAD51C and TP53. ...
by:West-Com Nurse Call System, Inc based inFairfield, CALIFORNIA (USA)
Connect is a VoIP, POE Nurse Call system designed especially for Acute Care facilities. It offers healthcare organizations powerful tools to improve and facilitate communication, collaboration, workflow, and patient safety. Facilities with our West-Call® and Novus® systems can upgrade to Connect without replacing patient room devices. Call us today to find out ...
Manufactured by:Kinamed Incorporated based inCamarillo, CALIFORNIA (USA)
Designed specifically for the small but challenging group of patients with isolated, end-stage patello-femoral disease, the KineMatch PFR (Patello-Femoral Replacement) offers a uniquely effective and conservative resurfacing solution. Because the device is precisely pre-fitted to the patient’s anatomy using CT (Computed Tomography) data, no ...
Manufactured by:Mizuho OSI based inUnion City, CALIFORNIA (USA)
Our latest modular table platform, offering enhanced safety features, improved setup workflows, and increased weight ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Cryptotanshinone is a natural compound extracted from the root of Salvia miltiorrhiza Bunge that shows antitumor activities. Cryptotanshinone inhibits STAT3 with an IC50 of 4.6 ...
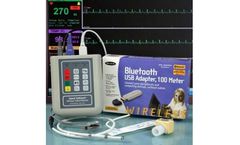
Manufactured by:Vmed Technology based inMill Creek, WASHINGTON (USA)
Now link your WiFi mobile device to our Windows software for remote monitoring and control of your wireless monitor. Click on the image below for overview and instructions on how to connect to mobile devices. Works for iOS, Android, Google Play, Microsoft and Blackberry ...