- Home
- Equipment
- north america
- postoperative patient
Refine by
Postoperative Patient Equipment Supplied In North America
6 equipment items found
Manufactured by:Adient Medical, Inc. based inPearland, TEXAS (USA)
Adient Medical is leading the development of an absorbable vascular filter for the prevention of pulmonary embolism (PE), a leading cause of death in the US claiming the lives of over 100,000 Americans annually - more than breast cancer, AIDS, and traffic fatalities combined. ...
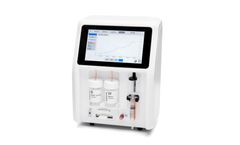
Manufactured by:Senzime AB Uppsala based inUppsala, SWEDEN
CliniSenz Analyzer is the future of postoperative and continuous patient monitoring in hospital environments. The system requires only small sample volumes for analysis, and CliniSenz Analyzer’s results are specific, with high precision. The Analyzer utilizes enzyme-based heat flows, which reduces the risk of interference from other compounds such as ...
Manufactured by:Entac Medical based inMemphis, TENNESSEE (USA)
Developing noninvasive devices for precision medicine based on novel, patented platform ...
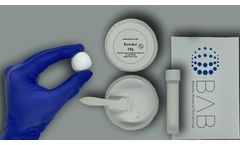
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Cem-Ostetic® is a neutral pH bone putty that contains biocompatible calcium salts that have been used for decades in orthopedic surgery. These materials are often used to provide an additional source of bone to help the patient heal faster. Berkeley Advanced Biomaterials formulates the chemistry and microstructure to enhance bone regeneration, provide optimal osteo-conduction, and ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Demineralized Bone Matrix (DBM) is produced from ground cortical bone and contains osteoinductive proteins. It is intended to be used to fill bone defects and ...
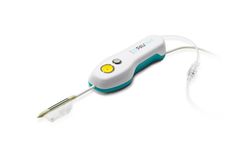
Manufactured by:PAVmed Inc. based inNew York, NEW YORK (USA)
We believe CarpX will have the potential to decrease costs by shifting the procedure from the operating room to a less expensive setting, reduce postoperative pain, accelerate the patient’s return to full activity, and lower the threshold for intervention for patients “suffering in silence” who chose to delay surgery until ...